41 electron configuration and the periodic table worksheet answers
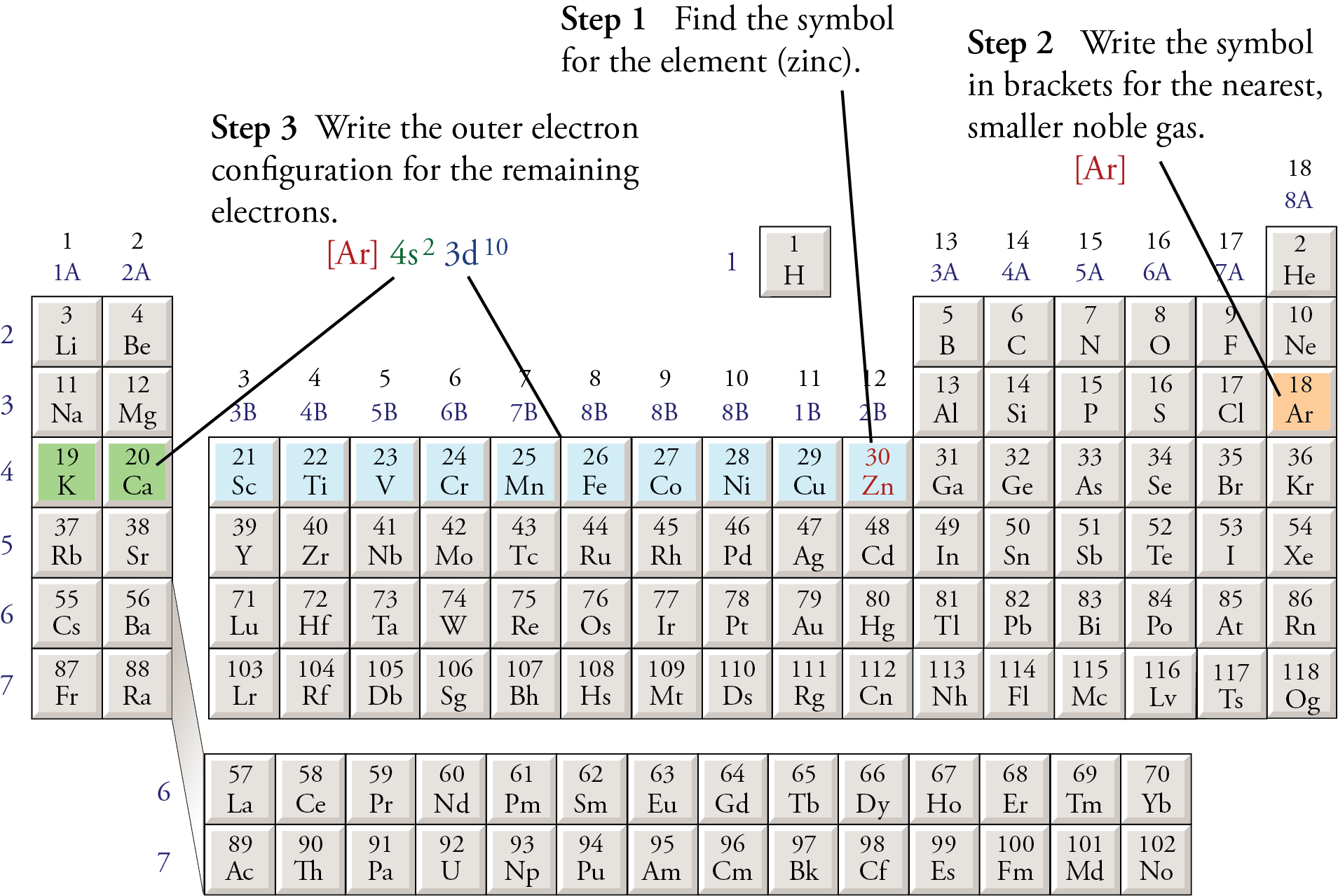
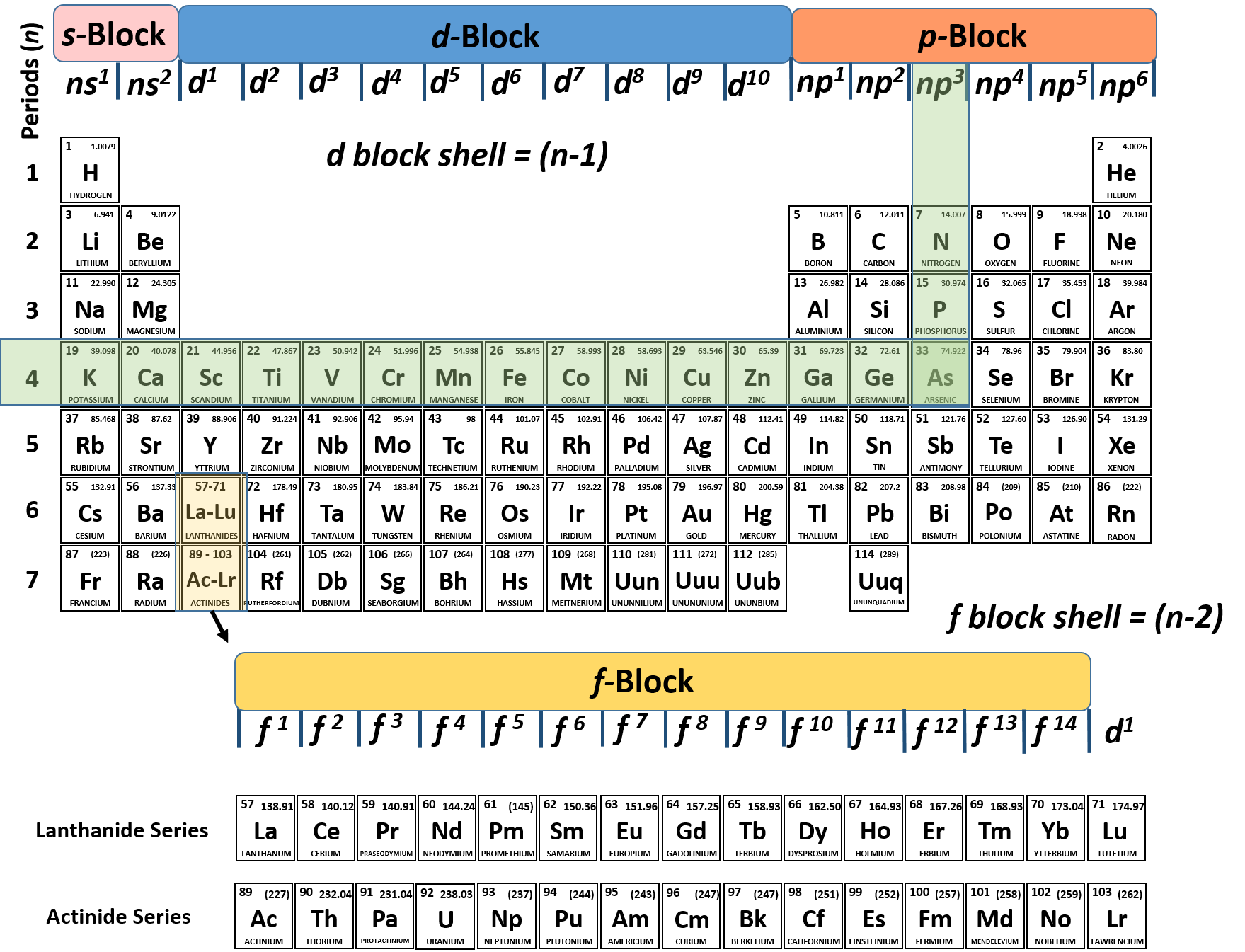
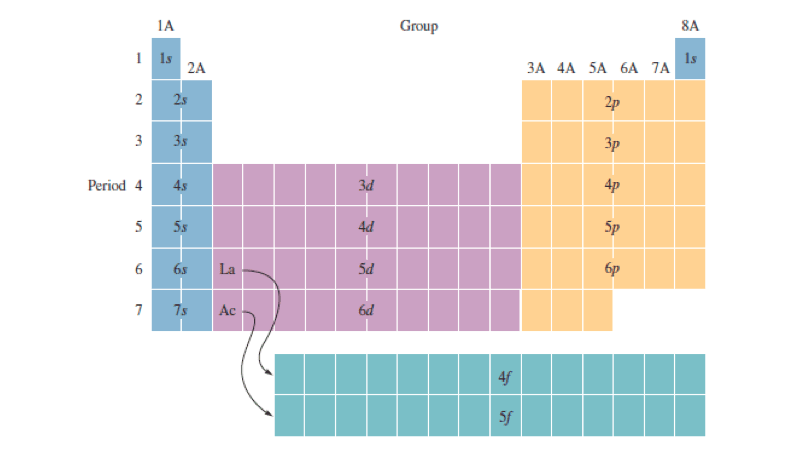
Information: Relating Electron Configurations to the Periodic Table In this section you will see how the periodic table serves as a road map for writing electron configurations. Get your periodic table out and get ready. Remember that a row on the periodic table goes horizontally from left to right. Columns are vertical (up and down).
As a result, these elements exhibit similar chemical behaviors and would be found in the same family or group on the periodic table. These elements are found in Group 2 or Family IIA of today's table. 3. Describe the electron configurations of the first twenty elements (Z = 1 to 20) using the s, p, d, f notation.
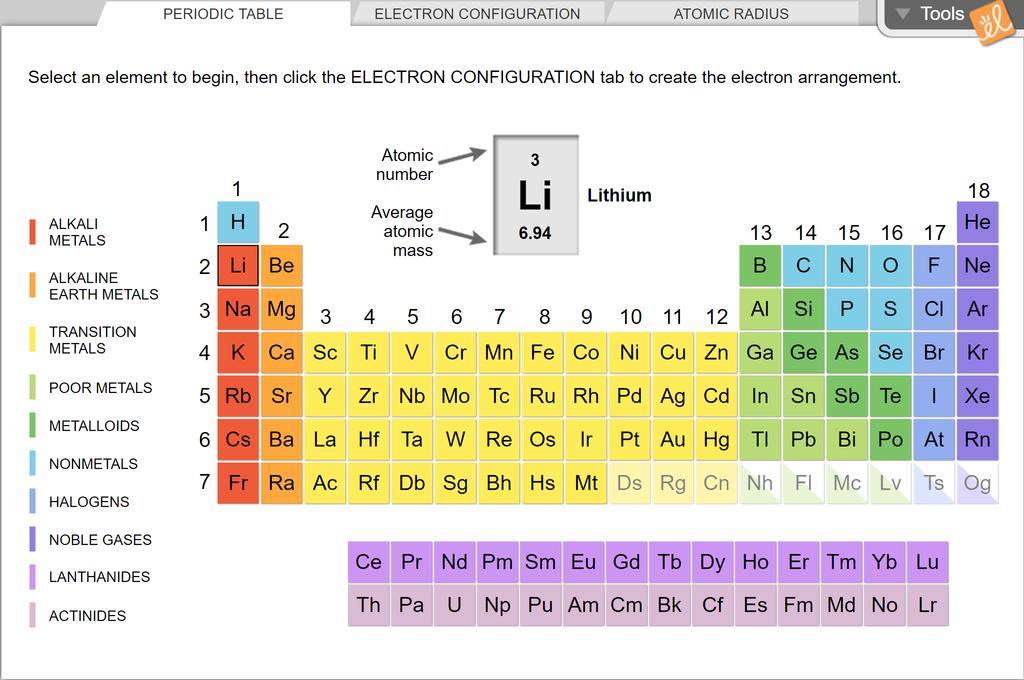
Electron Configuration Practice Chemistry How to write an electron confiquration: Name : Due Date: A. Determine the total number of electrons to be represented B. Use the Aufbau principle to fill the orbitals with electrons for elements 1-23. Refer to electron configuration periodic table for elements after 23 C.

Electron configuration and the periodic table worksheet answers
Ionization energies generally increase from left to right and decrease from top to bottom of a given group. Electron configuration is the arrangement of electrons around the nucleus of an atom based on their energy level. Electrons are added one at a time to the lowest energy levels first (Aufbau Principle).
50 Electron Configurations Worksheet Answer Key In 2020 Electron Configuration Geometry Worksheets Simplifying Algebraic Expressions . Details of using the periodic table as a guide for determining electron configurations can be found on the ch301 website. Electron configurations worksheet. Explain based on electron configuration why the noble ...
The answer is rather simple if you understand electron configurations. 51 53 62 63 85 electron configuration the periodic table print request for a color emr diagram you must fill out this form before the given date and time if you need a color emr diagram printed for you.
Electron configuration and the periodic table worksheet answers.
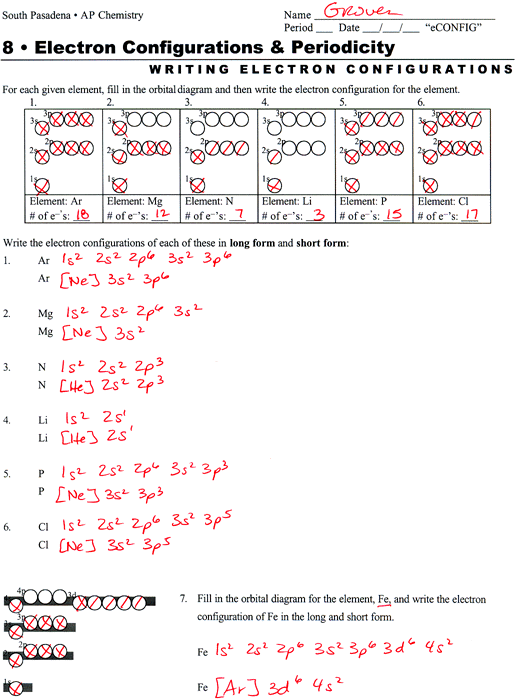
Answer: 1s2 2S2 Draw the electron configurations of the following atoms, ©lnstructional Fair, Inc. 3, 4, , Inco a O 29 ... The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this ...
Chemistry W.S.-Electron Configuration Practice 2 2014-15.docx Name _____ Date _____ Period _____ Electron Configuration Practice 2 PART A - ORBITAL DIAGRAMS & LONGHAND ELECTRON CONFIGURATIONS Use the patterns within the periodic table to draw orbital diagrams and write longhand
Part B: Use the patterns in the periodic table to electron configurations for the following atoms. Symbol Group # ... Part D: Use the info from #1-20 to answer the following questions. ... Electron Configuration Worksheet ...
• Associate groups on the periodic table with a number of valence electrons in an electron configuration. • Associate periods on the periodic table with a number of principle energy levels (shells) in an electron configuration. Success Criteria • Assign a group number for the elements in groups 1, 2 and 13-18 based upon the number
Electron configuration worksheet and lots more brief instructions an electron configuration is a method of indicating the arrangement of electrons about a nucleus. If your periodic table doesnt agree with this your answers for elements near the f orbitals may be slightly different. Can be a typical position job interview dilemma.
unreactive due to electron configuration •ns2np6 (except He 1s2) -Main group elements tend to gain or lose electrons to become isoelectronic (same valence electron configuration as nearest noble gas)
Electron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this, your answers for elements near the f-orbitals may be slightly different.
Stability & Electron Configuration - Ch. 4 CHEM PART B - SHORTHAND ELECTRON CONFIGURATION Use the patterns within the periodic table to write the longhand electron configuration notation for the following elements. Symbol # e- Longhand Electron Configuration Notation 7. S 1s2 2s2 2p6 3s2 3p4 8. Pb 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 ...
8. The alkali metals have a single electron in the highest energy level. 9. The alkaline earth metals achieve the electron configurations of noble gases by losing 2 e- 10. The transition metals vary in the number of electrons in the highest energy level 11. The halogens achieve the electron configuration of noble gases by gaining one electron. 12.
Electron Configuration Worksheet Answers 3 Electron Configuration Worksheet Pdf In 2020 Electron Configuration Practices Worksheets Chemistry Worksheets , Table & E Configuration Practice Test Multiple Choice Identify The Choice That Best Completes The Statement Or Answers The Question.
1. Fill in the electron configurations for the elements given in the table. Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3.
The Periodic Table - Worksheet - ANSWERS. Which has a larger radius, Na+ or Ne? Briefly explain. Ne. Both have the same number of electrons filling the same orbitals. However, Ne has less nuclear charge thus leading to a weaker attraction to the outer electrons. Identify the three elements using the information listed below: Element X: Sulfur, S.
Use the patterns within the periodic table to write the shorthand electron configurations for the following elements. Symbol # e- Shorthand Electron Configuration 13. Ca 14. Pb 15. F 16. U PART D - RULES OF ELECTRON CONFIGURATIONS Which of the following "rules" is being violated in each electron configuration below? Explain your answer ...
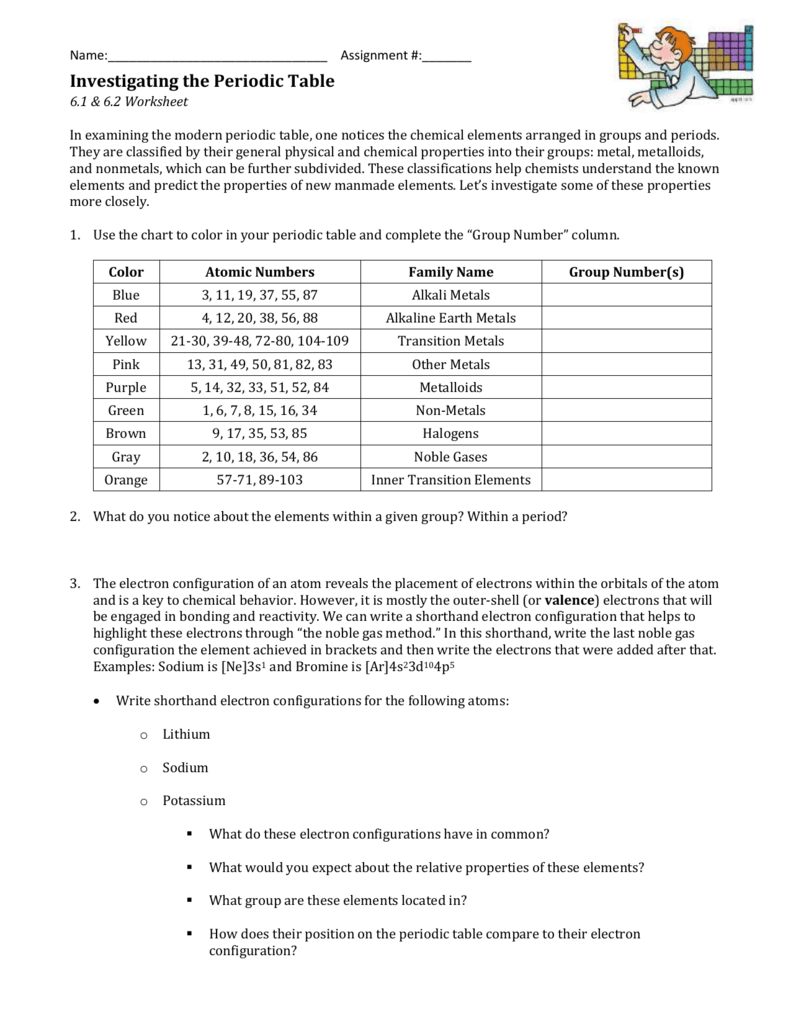
Periodic Table WS #2 KEY . 1. How many elements are listed in the periodic table? (the one Dr. Hart gave you…) 118 2. What is the atomic number of selenium? ... What can be said about the electron configurations of all the elements in a group? _their valence electron configurations are identical_____ The s-, p-, d-, and f-Block Elements .
Electron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this, your answers for elements near the f-orbitals may be slightly different.
(Brief periodic table location description for each clue) Clue #1) I have a high electron affinity, (highly negative value), and my atomic number is X. HALOGEN FAMILY Clue #2) The element with atomic number X-1 has a lower ionization energy and a lower electron affinity.
The 6 key answers for the electron configuration chem worksheet 5 are. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide.
This worksheet provides extra practice for writing electron configurations. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. Details of using the periodic table as a guide for determining electron configurations can be found on the CH301 website. 1.
Electron configuration and the periodic table worksheet answers How to draw an electron configuration diagram. Find the element on the periodic table. The atomic number tells you how many electrons to draw in total. For example, potassium has 19 electrons. Draw a small circle and write the symbol in the centre. This represents the nucleus.
Electron Configurations - Solutions Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn't agree with this, your answers for elements near the f-orbitals may be slightly different.
Unit 2 worksheet 2: Valence electrons and worksheet 2 answers p.162 #27-28 Homework: Finish worksheet 2 Lesson 5(Thursday 19th September): Electron configurations and the periodic table The periodic table notes Unit 2 Worksheet 3: The periodic table p.186 #8, 10, p.199 #54-56 Lesson 6 (Monday 23rd September): Ions
The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. The 6 key answers for the electron configuration chem worksheet 5 are. Or pull onto electrons is known as electronegativity this can be related to electron configuration worksheet 2 answer key we are living in an era of.
Electron Configurations Worksheet with Answers Videos Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. Electron Configurations Video Worksheet





























0 Response to "41 electron configuration and the periodic table worksheet answers"
Post a Comment