41 types of chemical equations worksheet
30/11/2021 · This systems of equations worksheet is a good resource for students in the 5th Grade 6th Grade 7th Grade and 8th Grade. All worksheets created with Infinite Algebra 1. Gain immense practice with this batch of printable solving systems of equations worksheets designed for 8th grade and high school students. And there is nothing like a set of co-ordinate axes to …
8th Balancing Equations Worksheet I Balance the following chemical equations 1. 2 K + 2 H 2 O 2 KOH + H 2 2. 3 MnO 2 + 4 Al 3 Mn + 2 Al 2 O 3 3. 2 Al 2 O 3 4 Al + 3 O 2 4. 3 H 2 + P 2 2 PH 3 5. 4 Fe + 3 O 2 2 Fe 2 O 3
Chemical Equation Worksheet. NAME: Types of Reactions: Identifying and predicting products. For each reaction equation: 1. Balance the equation.2 pages

Types of chemical equations worksheet
Balance the equation. 2. Identify the type of reaction (synthesis, decomposition, single displacement, double displacement or combustion). Reaction Type.
Types of Reactions Worksheet – Solutions Balance the following equations and indicate the type of reaction taking place: 1) 3 NaBr + 1 H3PO 4 1 Na 3PO 4 + 3 HBr Type of reaction: double displacement 2) 3 Ca(OH) 2 + 1 Al 2(SO 4)3 3 CaSO 4 + 2 Al(OH) 3 Type of reaction: double displacement 3) 3 Mg + 1 Fe 2O3 2 Fe + 3 MgO Type of reaction: single displacement 4) 1 …
Balancing Chemical Equations – Worksheet #3 Write balanced chemical equations for each of the following and then classify each reaction as a synthesis, decomposition, single-replacement, double replacement, acid-base reaction, or combustion. Be aware that some reactions may fall into more than one category. Balanced Equation Rxn Type
Types of chemical equations worksheet.
This process always involves energy Chemical reactions are represented by sentences known as chemical equations. A . chemical equation. describes exactly what happens in a chemical reaction. A . chemical equation. shows compounds before a chemical reaction takes place on the left (reactants) and compounds formed from the chemical reaction on the right (products). …
After working on this worksheet, you should be able to do the following: 1). Given an equation, you should be able to tell what kind of reaction it is.6 pages
Name the type of reaction: a. Magnesium + oxygen → Magnesium oxide. Oxidation b. Copper carbonate → copper oxide + carbon dioxide. Thermal decomposition.3 pages
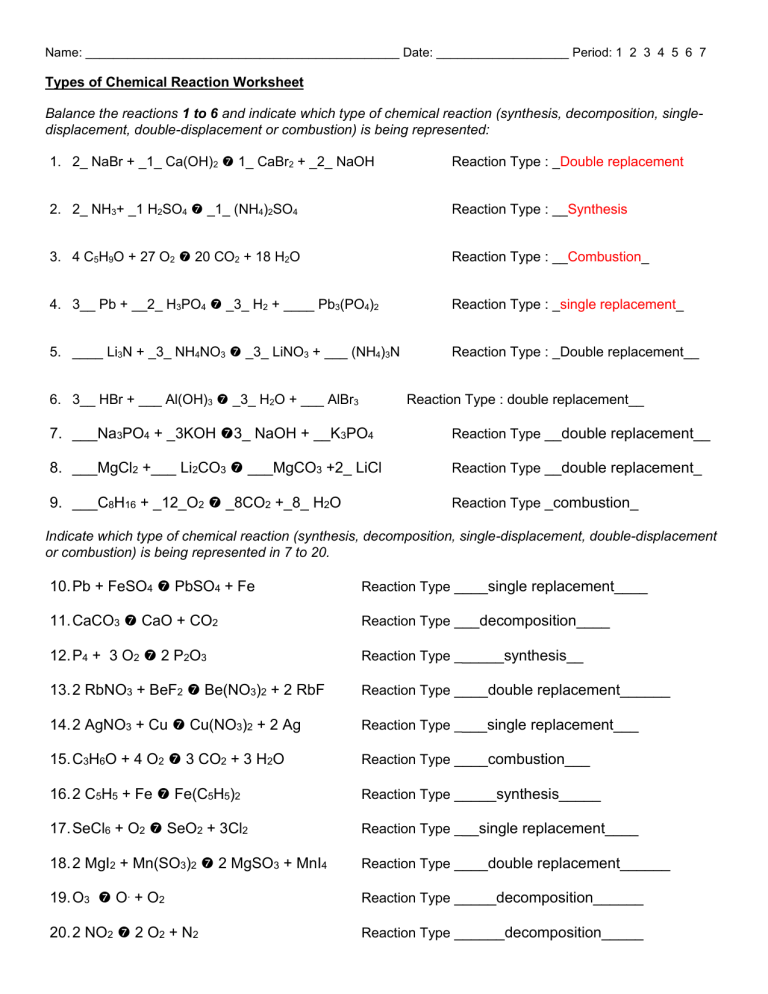
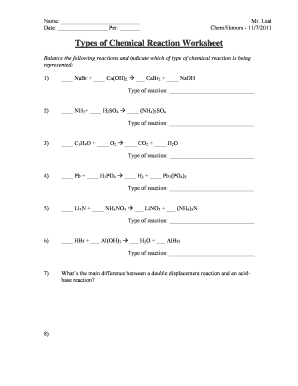
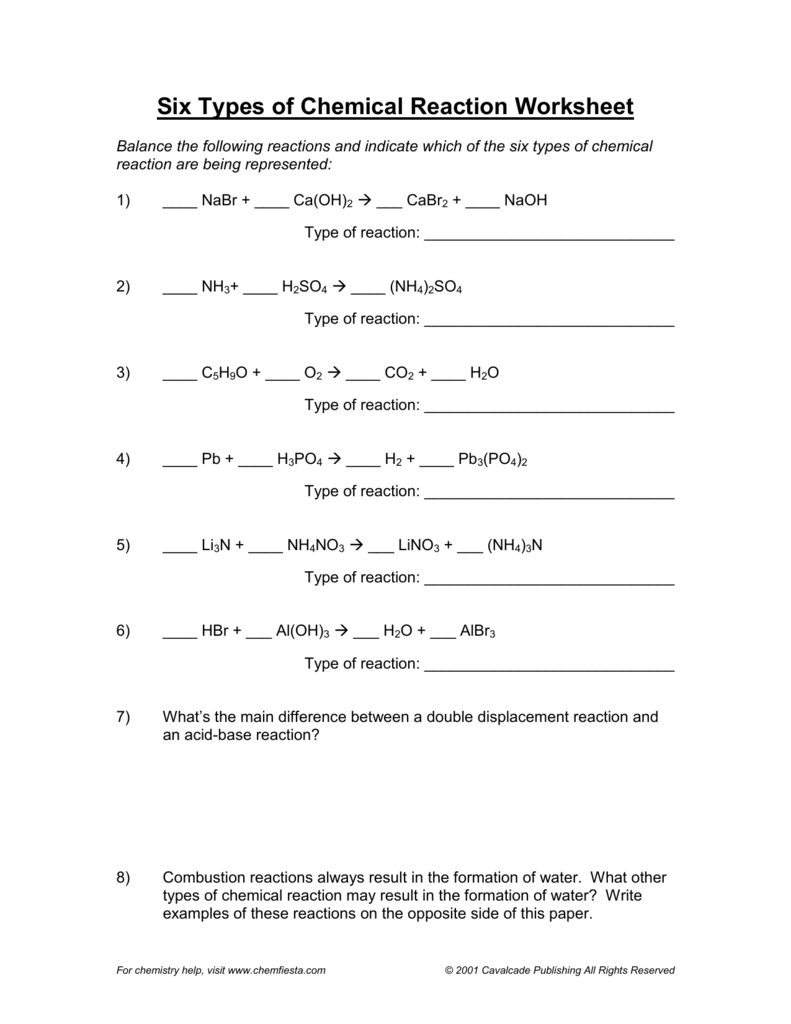
Types of Chemical Reactions. Balance each of the following reactions and identify each type of reaction: 1. ____ NaBr + ____ Ca(OH)2 → ___ CaBr2 + ____ ...
(Note that oxidation and reduction are shown as separate classes.) Resources s Student worksheet. – Types of chemical reaction. Feedback for students.
02/04/2020 · Basically, there are five types of chemical equations and their reactions. Check them out below. Combination or Synthesis Chemical Reaction. This is the most common type of chemical equation. In this chemical equation, a new product is formed by combining two to three combinations of reactants. For instance, H 2 + O 2 H 2 O. This is a chemical equation where …
04/02/2021 · TemplateLab: 49 free balancing chemical equations worksheet downloads . Balancing Chemical Equations: Key Takeaways . Balancing chemical equations seems complicated, but it’s really not that hard! Your main goal when balancing chemical equations is to make sure that there are the same amount of reactants and products on each side of the …
The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion. Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. Some reactions will fit into more than one category. Combination Reactions. A combination reaction, also known as a synthesis reaction, …
Types of Chemical Reactions Answers Balance each of the following reactions and identify each type of reaction: 1. 2 NaBr + Ca(OH) 2 CaBr 2 + 2 NaOH double displacement 2. 2 NH 3 + H 2 SO 4 (NH 4) 2 SO 4 synthesis 3. 4 C 5 H 9 O + 29 O 2 20 CO 2 + 18 H 2 O combustion 4. 3 Pb + 2 H 3 PO 4 3 H 2 + Pb 3 (PO 4) 2 single displacement 5. Li 3 N + 3 NH 4 NO 3 3 LiNO 3 + (NH …
Worksheet 6.2 Word Equations 1. Write the chemical equations and balance each of the following word equations. a) Aluminum metal reacts with iron (II) oxide powder to produce aluminum oxide solid and iron metal. _____ b) Aluminum sulphate solution and calcium hydroxide solution produce a precipitate of aluminum hydroxide and solid calcium sulphate. _____ c) …














![Types Of Chemical Reaction Worksheet Practice Answers [z06w3koy3jqx]](https://doku.pub/img/crop/300x300/z06w3koy3jqx.jpg)















![49 Balancing Chemical Equations Worksheets [with Answers]](https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-41.jpg)




0 Response to "41 types of chemical equations worksheet"
Post a Comment