38 atomic theory review worksheet answers
Nov 15, 2021 · Modern atomic theory understands electrons as operating more similarly to a cloud than the previously-theorized planetary model. Learn the history of atomic theories through the Bohr model ... _____1. Democritus’s original atomic theory was revised because it a. claimed matter is made of atoms. c. explained what electrons are. b. claimed atoms could be divided. d. did not have a scientific basis. _____2. Dalton’s atomic theory stated that every element was made of atoms that could not be subdivided, atoms of the same element are ...
Dec 06, 2021 · Vocabulary review and key 8. Basic atomic structure worksheet h and the 1. Understanding the atom finding numbers of protons neutrons electrons and key 10. The content and theme of this. Chapter 4 atomic structure worksheet answers core teaching. View 3 06a atomic structure wkst key pdf from aa 1worksheet. Prcions ð o their respective charges are.
Atomic theory review worksheet answers
Title: Quantum Theory of the Atom Worksheet Author: rhardi Last modified by: Rosa Created Date: 9/13/2011 5:32:00 AM Other titles: Quantum Theory of the Atom Worksheet Answer Q 20, 21 and 22 on page 35 6 Note: Nuclear Chemistry (cont.) Half-lives of Radioisotopes Quiz #1 Questions 12 and 13 on page 32 of text Begin Review Questions for Atomic Theory 7 Note: Summary of Atomic Structure, so far ... PLAY. More than 2000 years ago, Greek philosophers proposed the existence of very small, indivisible particles, each of which is called an. The theory that such particles existed was supported, much later by , who proposed, in his law of , that matter can't cannot be created or destroyed. Nice work!
Atomic theory review worksheet answers. Atomic Theory Worksheet No 2 (Atomic Theory Worksheet 2 Answer Key.pdf) Atomic Theory Worksheet No 3 (Atomic Theory Worksheet No 3.pdf) Atomic Theory Worksheet No 4 (Atomic Theory Worksheet No 4.pdf) ... Atomic Theory Unit Review Worksheet (U6_RevWS_Key.pdf) Periodic Table. Chapter 11 Worksheet ... The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers. The atomic mass (atomic weight) of an element is weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes. Carbon: Atomic Number and Atomic Mass 12C 6 AC Z 13C 6 (98.9 12.000) (1.1 13.000) 12.011 100 × +× = 1 Atomic Structure from s3.studylib.net. Experimental observations that led scientists atomic structure this worksheet and all related files are licensed under the creative commons answers to review questions for atomic theory quiz #1 multiple choice questions: Development of the atomic theory directions: Fill in the blanks on the right with the information in the chart below.
Dalton's atomic theory stated that atoms separate, combine, or rearrange in chemical reactions. Dalton's atomic theory stated that matter is mostly empty space. 5. Dalton was correct in thinking that atoms could not be divided into 6. smaller particles. Dalton's atomic theory stated -that atoms of different elements combine In How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table. Molar Mass Calculations. 1, 22. worksheet answers, hr diagram sheet hr diagram student mini lab name jason, hertzsprung limited to the different kinds of spectrum spectral classification and temperature problem to create a hertzsprung atomic mass isotopes atomic mass phet, near atomic map of parathyroid hormone complex points, development of ... Aug 22, 2021 · The current knowledge of atoms and atomic theory has been informed by many scientists going back to Aristotle and Democritus. Learn about the contributions made to early atomic theory by ...
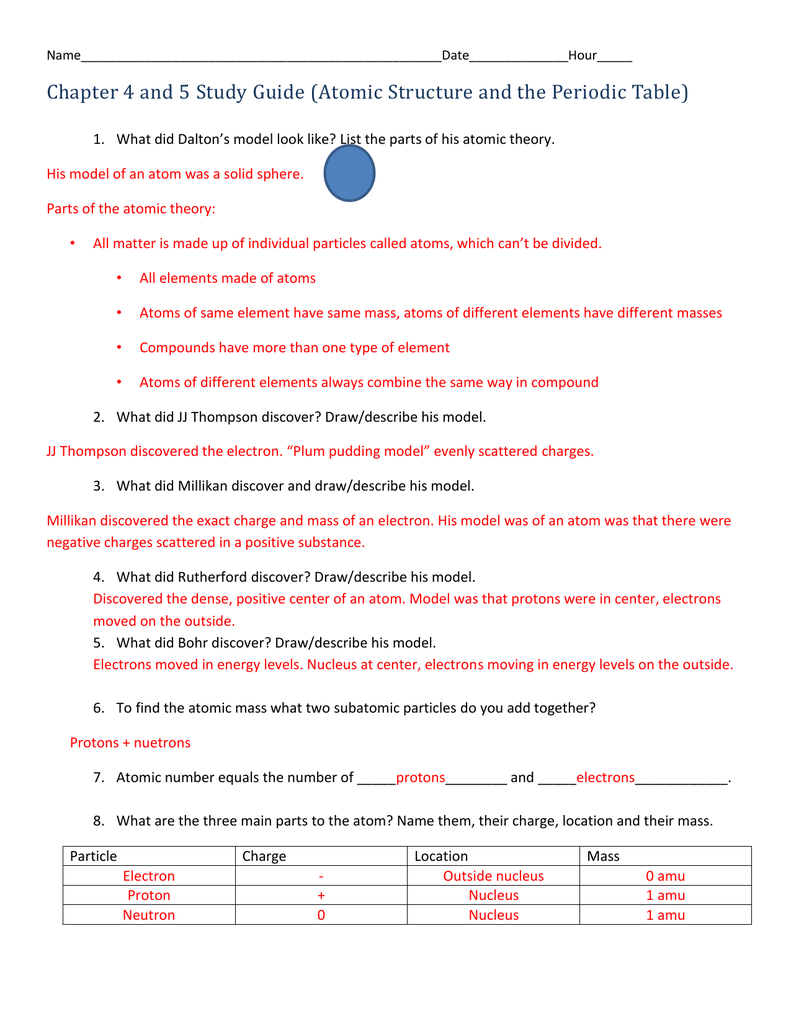
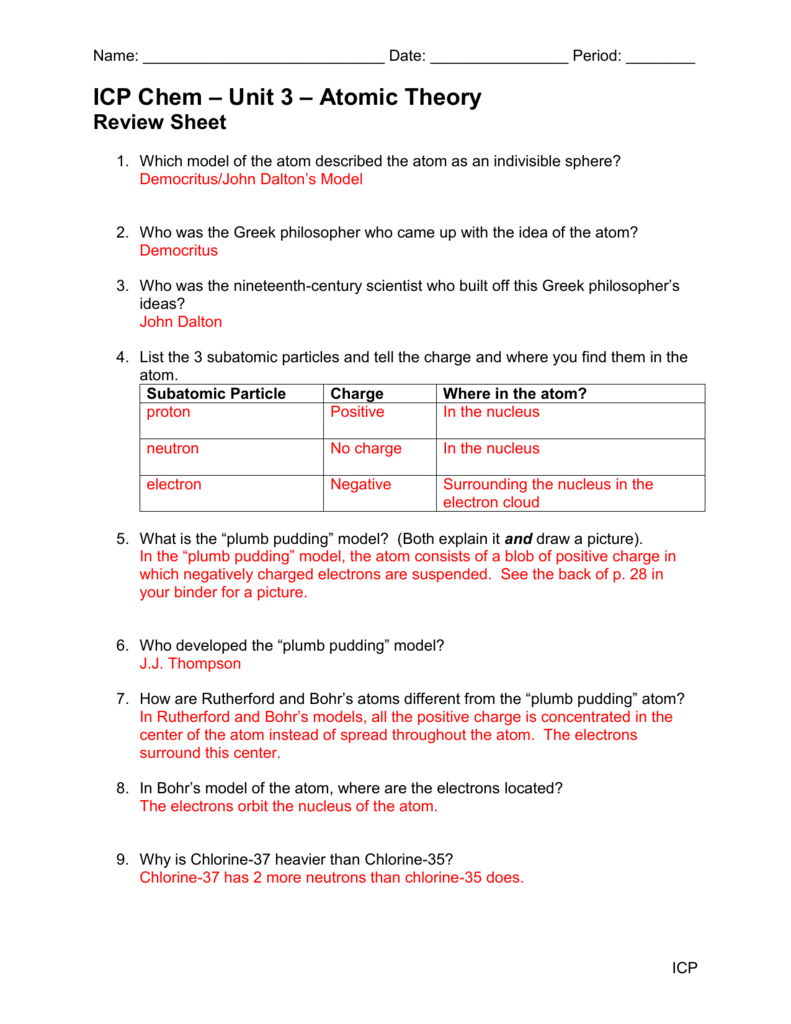
Q. What contribution did John Dalton make to atomic theory? answer choices He discovered that every atom was positively charged. He discovered that every element consisted of one type of atom. He discovered that atoms had nuclei. He discovered that atoms could be divided into smaller parts. Question 14 120 seconds 3—3 Review and Reinforcement Modern Atomic Theory Complete the following table. 1. 2. 3. If the statement is true, write “true.” If it is false, change the underlined word or words to make the statement true. Write your answer on the line provided. 4. Moseley discovered that all atoms of an element have the same number of neutrons in ... Worksheet development of atomic theory answers Oxygen l 6 8 16 30 8 8 8 3 States that the masses of one element that combine with a fixed mass of another element in different compounds are in simple whole number ratios It will show the progression about the best way to solve the problems Carbon 14 6 14 146c 6 8 6 2 Get the free atomic theory practice worksheet answers form Description of ... , CHAPTER 6 REVIEW ACrlVlTY Text Reference: Section 6-90 Development of Atomic Theory Choose words from the list to fill in the blanks in the paragraphs. Word List atom atomic number Bohr Chadwick conservation of matter Dalton definite proportions electron energy level isotope Lavoisier mass number 1. multiple proportions neutron 2. nucleus
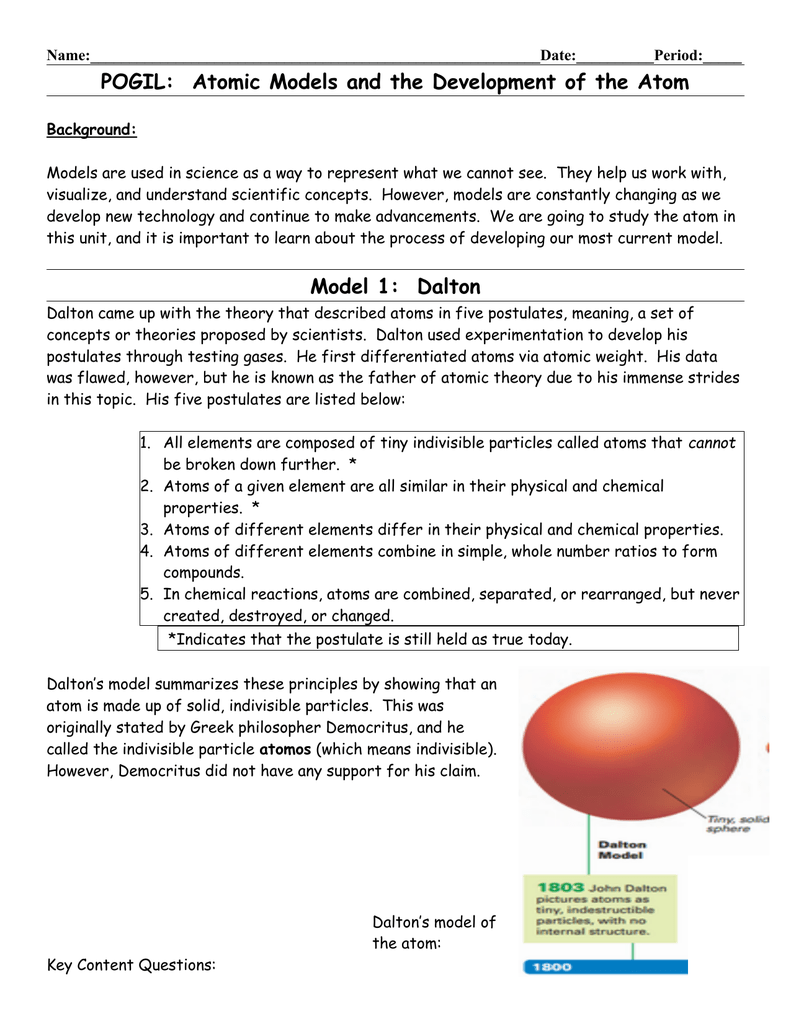
Some of the details of Dalton's atomic theory require more explanation.. Elements: As early as 1660, Robert Boyle recognized that the Greek definition of element (earth, fire, air, and water) was not correct. Boyle proposed a new definition of an element as a fundamental substance, and we now define elements as fundamental substances that cannot be broken down further by …
The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.
PLAY. More than 2000 years ago, Greek philosophers proposed the existence of very small, indivisible particles, each of which is called an. The theory that such particles existed was supported, much later by , who proposed, in his law of , that matter can't cannot be created or destroyed. Nice work!
Answer Q 20, 21 and 22 on page 35 6 Note: Nuclear Chemistry (cont.) Half-lives of Radioisotopes Quiz #1 Questions 12 and 13 on page 32 of text Begin Review Questions for Atomic Theory 7 Note: Summary of Atomic Structure, so far ...
Title: Quantum Theory of the Atom Worksheet Author: rhardi Last modified by: Rosa Created Date: 9/13/2011 5:32:00 AM Other titles: Quantum Theory of the Atom Worksheet






















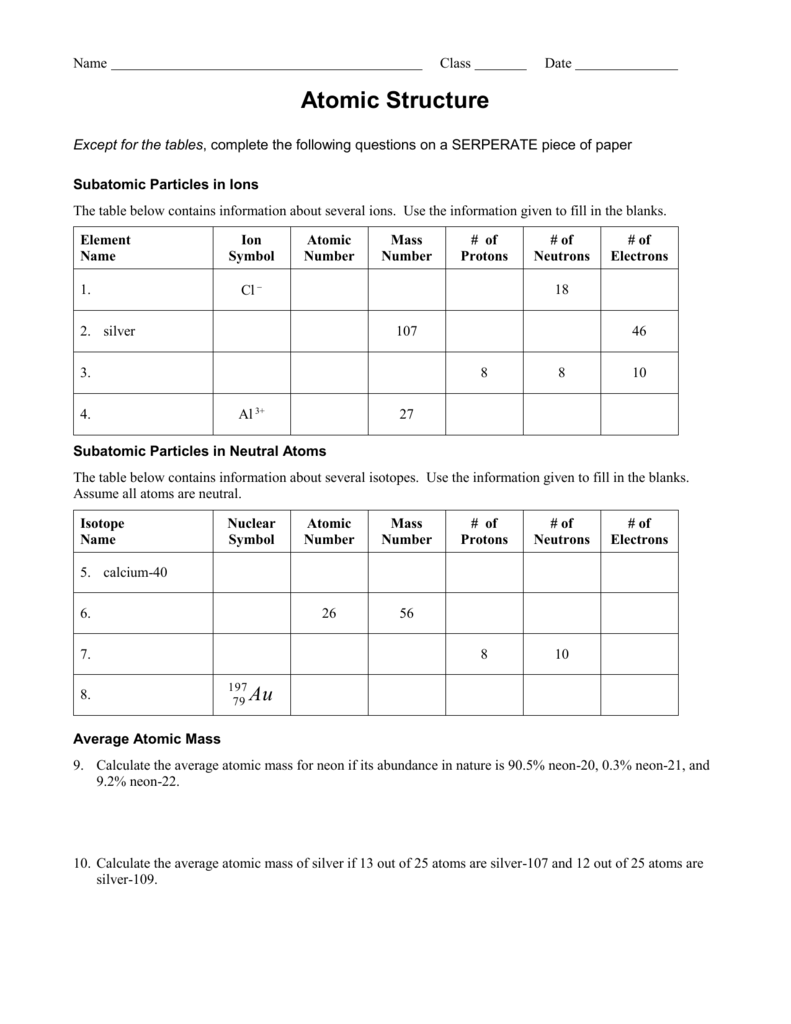






0 Response to "38 atomic theory review worksheet answers"
Post a Comment