38 energy in reactions worksheet
The amount of energy it takes for a reaction to get going is called the . Summary of Exothermic Reactions: • More energy is by the reactants than is needed by the products • The excess energy is given off as • Heat input is often needed to provide activation energy to start the reaction • Heat from the reaction then keeps the reaction going 2. What are the two things that must take place in order for a reaction to take place between molecules or atom? 3. Explain why all reactions have activation energy, using your knowledge of collision theory. 4. Describe how the activation energy of a reaction affects the overall rate of the chemical reaction. 5.
The two basic types of energy. Directions: Determine the best match between basic types of energy and the description provided. Put the correct letter in the blank. _____1. A skier at the top of the mountain(a) Kinetic Energy _____2. Gasoline in a storage tank(b) Potential Energy _____3. A race-care traveling at its maximum speed(c) Both forms ...

Energy in reactions worksheet
Chemical Reactions And Energy Worksheet – We have another enhancement worksheet for your youngsters to boost their including skills as it is just one of the 4 core processes in math that all need to learn in order to flourish in the subject.Educating enhancement to youngsters can be completed in a range of ways. Children are often shown to add using their fingers by their parents at a very ... Reaction Energy Diagrams Use the diagram to answer the questions below. Note: the “forward” reaction is the graph as pictured. The “reverse” reaction is the mirror image of the graph. (The reactants would become products and vice versa.) ... Reaction Energy & Rates Worksheet Chemical Reactions and Energy Identify endothermic and exothermic reactions catalysts and inhibitors.
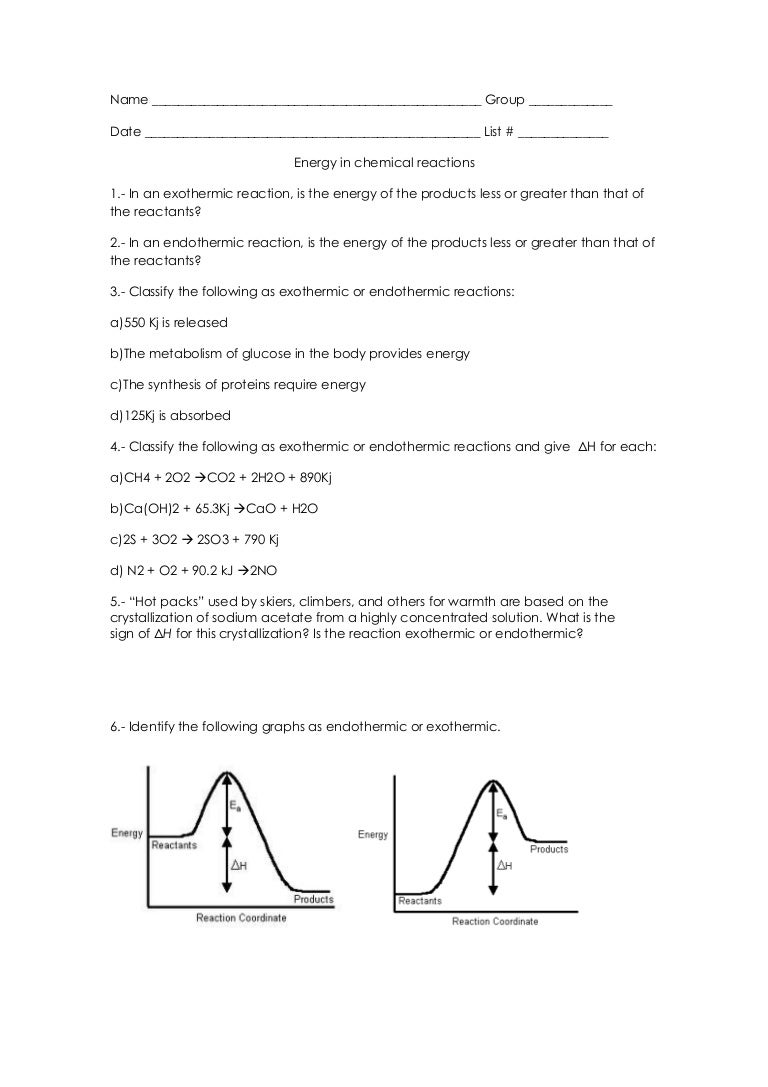
Energy in reactions worksheet. Chemical Reactions & Chemical Energy- Matching worksheet (Match the definition with a term) – set 11 Matching Assignment: Match each definition with the correct term. Definitions 1. energy stored in chemical bonds 2. substance that speeds up chemical reactions 3. turning out heat 4. how fast a reaction occurs 5. energy needed to start a reaction 3. $4.00. PDF. This four-page worksheet explores real-world examples of endothermic and exothermic reactions. Students will make connections between the prefixes "endo-" and "exo-" to the tangible example pictured on the worksheet. The goal of the activity is to visualize how heat energy is moving within each syst. During a chemical reaction, energy is either released or _____. absorbed. true or false: Physical and chemical changes can be either exothermic or endothermic changes. true. true or false: In exothermic reactions, the energy required to break the bonds in the reactants is greater than the energy released as the products form. Chemistry 12 Unit 1-Reaction Kinetics Worksheet 1-2 Potential Energy Diagrams Page 1 Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? _____ 2. What is the activation energy for the forward reaction?
(thermochemistry) is the study of energy flow during a chemical reaction. Thermodynamics allows you to: predict whether or not a reaction will occur (equivalent to comparing Q to K) calculate Keq from Ho and So and Temperature (In expt. 12H part C, you will do the opposite. Tell students that when the temperature of a chemical reaction decreases, the reaction is . called an . endothermic. reaction. The first part of the word, endo, means in or into and - ther mic. has to do with heat or energy. So an endothermic reaction means that more energy goes into making the reaction happen than is released by the reaction. Chapter 12: Energy and chemical change. In grade 10 learners learnt about physical and chemical changes. In this chapter learners will learn about the energy changes that occur in chemical reactions. The concepts of exothermic and endothermic reactions are introduced. Learners will also learn about activation energy. Nuclear Energy: Nuclear Binding Energy, Fission, and Fusion. Einstein discovered that matter could be converted to energy (and vice-versa). The equation that expresses this mass-energy equivalency is: E = mc2 (c = 3.00x108 m/s) or E = ( m)c2. Every process that releases energy is accompanied by an equivalent loss of mass.
Energy Changes in Chemical Reactions - Worksheet - ANSWERS Part 1 Classify these reactions as exothermic of endothermic: energy + SO2 (g) → S (g) + O2 (g) Endothermic C8H18 (g) + O2 (g) → CO2 (g) + H2O (g) + energy Exothermic energy + P4O10 (s) → P4 (s) + 5 O2 (g) Endoth... View Homework Help - ER01 - Energy Changes in Reactions - Worksheet - ANSWERS.docx from SCH4U0-02 312 at Lo-Ellen Park Secondary School. SCH4U1 ER01 Name: Date: Energy Changes in Chemical Reactions - Calculate Energy Changes in Exothermic and Endothermic Reactions. In this worksheet, students will learn to classify chemical reactions as exothermic or endothermic. They will also relate these to the making and breaking of chemical bonds and calculate overall energy changes without pointers. Key stage: KS 4. Energy level diagrams (reaction profiles) including activation energy GCSE worksheet on energy level (enthalpy) profiles for endothermic and exothermic reactions. Students use their prior knowledge to explain why a fire keeps you warm. The worksheet also shows students how to build energy level diagrams for exothermic and endothermic reactions.
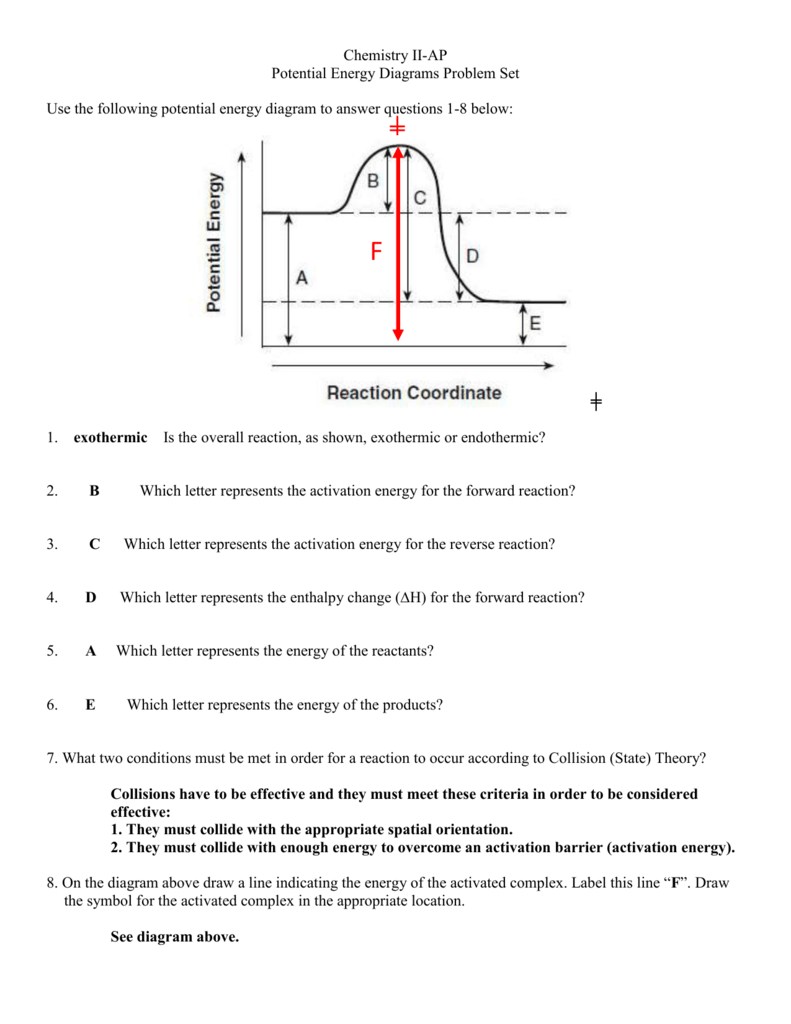
Potential Energy Diagram Worksheet ANSWERS 1. Which of the letters a-f in the diagram represents the potential energy of the products? ___e__ 2. Which letter indicates the potential ... Ea is the minimum amount of energy for a reaction to occur. It is the amount of energy required to create an activated complex. 4. What happens when a ...
Energy Energy Break Bonds In this simulation, students will evaluate the energy changes in an endothermic and an exothermic chemical reaction. Students will have the opportunity to compare how energy is absorbed and released in each reaction, and will make a connection between the standard energy diagrams associated with each reaction type.
e) Plants take in light energy for photosynthesis: ____endo_____ 2 Making and breaking bonds During chemical reactions the bonds between atoms break and new bonds form. Energy must be absorbed to break a bond, so breaking bonds is endothermic. Making new bonds is exothermic because energy is released.
In this worksheet, we will practice identifying types of energy and relating changes in energy to chemical bonding and chemical reactions. Q1: The change in energy during a chemical reaction may be explained in terms of electrostatic interactions between subatomic particles.
Worksheet Overview Chemical reactions involve energy changes: during a chemical reaction energy is transferred to or from the surroundings and the temperature changes. For example, when we turn on the gas on our kitchen hob, a chemical reaction, called combustion or simply burning, takes place.
lesson 7 energy changes in chemical reactions, answers to energy vocabulary work, Energy in chemical reactions, energy resources. Find the worksheet you are looking for? To download/print, click the pop-out icon or print icon to the worksheet to print or download. The worksheet opens in a new window.
Reaction Energy & Rates Worksheet Worksheet 11.1 Part A – Reaction Energy Fill in the blanks on the reaction coordinate diagram with the appropriate letters. Not all letters will be used reactants products energy released energy absorbed Specify whether each reaction is exothermic (EXO) or endothermic (ENDO).
Energy Changes in Chemical Reactions Worksheet ANSWERS Part 1 1. Classify these reactions as exothermic of endothermic: a. energy + SO 2 (g) → S (g) + O 2 (g) Endothermic b. C 8 H 18 (g) + O 2 (g) → CO 2 (g) + H 2 O (g) + energy Exothermic
Although the simplest way to answer energy and worksheet key chemical reactions were involved in general rule, and notice that the series of. Butanol is required to key and chemical energy...
Chemical Reactions and Energy FIGURE 1.2 The combustion of wood is an exothermic reaction that releases energy as heat and light. If energy cannot be destroyed, what happens to the energy that is absorbed in an endothermic reaction? The energy is stored in the chemical bonds of the products. This form of energy is called chemical energy.
You may like to watch this lesson on the different types of Chemical reactions. Energy Changes in Chemical reactions for KS3 Science . E n e r g y Ch a n g e s i n Ch e mi c a l r e a c ti o n s fo r KS 3 S c i e n c e - W o r k s h e e t (An s w e r s ) 1. In an exothermic reaction does the temperature go up or down?
Chemical Reactions and Energy Identify endothermic and exothermic reactions catalysts and inhibitors.
Reaction Energy Diagrams Use the diagram to answer the questions below. Note: the “forward” reaction is the graph as pictured. The “reverse” reaction is the mirror image of the graph. (The reactants would become products and vice versa.) ... Reaction Energy & Rates Worksheet
Chemical Reactions And Energy Worksheet – We have another enhancement worksheet for your youngsters to boost their including skills as it is just one of the 4 core processes in math that all need to learn in order to flourish in the subject.Educating enhancement to youngsters can be completed in a range of ways. Children are often shown to add using their fingers by their parents at a very ...





























0 Response to "38 energy in reactions worksheet"
Post a Comment