38 molecular mass and mole calculations worksheet answers
Mole calculation practice. Molar mass practice. Pin On Examples Worksheet Answers Key Find the formula mass of the following compounds. Chemistry molar mass worksheet answer key. Displaying top 8 worksheets found for molar mass and answer key. Learn important chemistry concepts like chemical equations mole and molar mass chemical formulas mass relationships in equations limiting […] Mole Calculations involving Masses of solids m m = the mass of the substance given in the question n gfm n = number of moles gfm = the mass of 1 mole of the substance Example How many moles are present in 25g of CaCO 3? n = m gfm n = ? CaCO 3 m = 25g 3 x 16 = 48 gfm = 100g 1 x 12 = 12 1 x 40 = 40 100g
Molecular mass and mole calculations worksheet answers. 2141 grams 5 how many moles are in 2 3 grams of phosphorus.Mole Calculations Review Worksheet answers on next page. Students will learn simple techniques on how to solve problems involving empirical formula and molecular formula from this worksheet.

Molecular mass and mole calculations worksheet answers
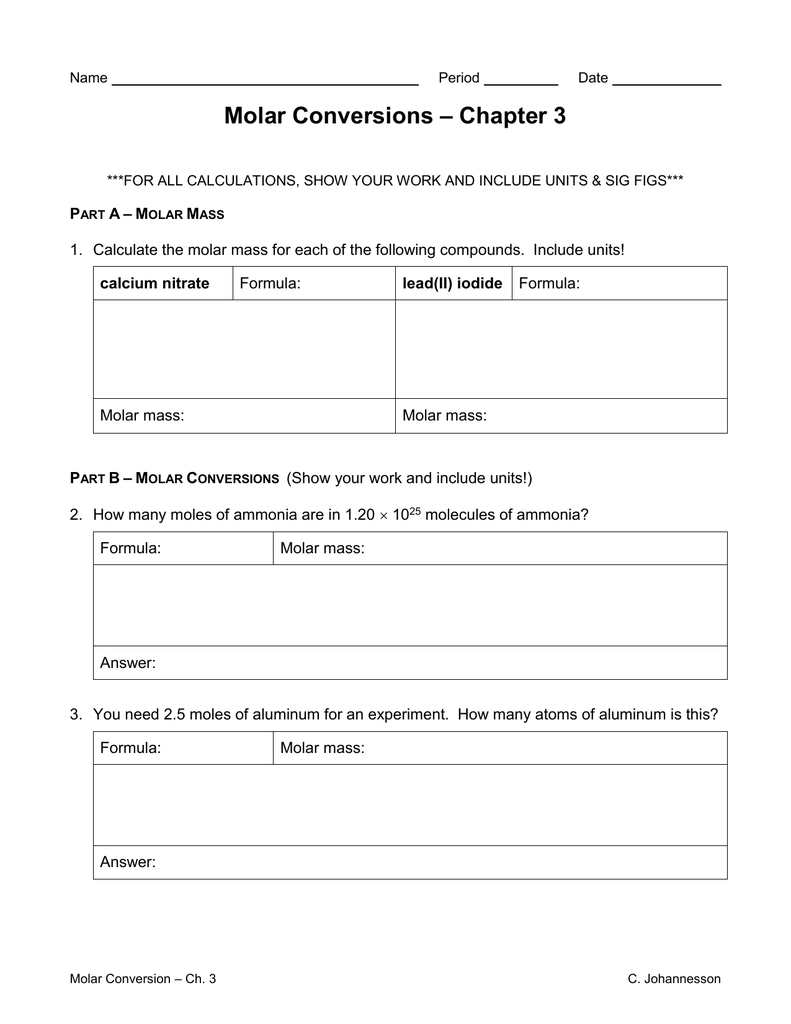
Exercise 4d Calculation of the number of moles of gas in a given ... Answers 115 . UA008883 - Workbook for GCE students - Moles, ... Relative Molecular Mass of a molecule is calculated by adding together the relative atomic masses of the atoms in the chemical formulae. Worksheet 11.1 KEY Molar Mass Calculations A mole is a standard unit of measurement for amount of a substance. For an element, one mole is equivalent to the mass of that element as it is listed on the periodic table, and thus represents its molar mass. For a compound, one must take the sum of all elements in the compound with Mole Calculations Review Worksheet - answers on next page. 1. Calculate the molar mass of each compound. a. LiOH c. Mg(C 2H 3O 2) 2. b. barium bromide d. Ca(NO 3) 2. 2. How many molecules are in 45.0 grams CH 4? 3. How many moles are in 18.8 grams NaOH? 4. A salt 23shaker containing 9.58 x 10 formula units NaCl contains how many moles? 5.
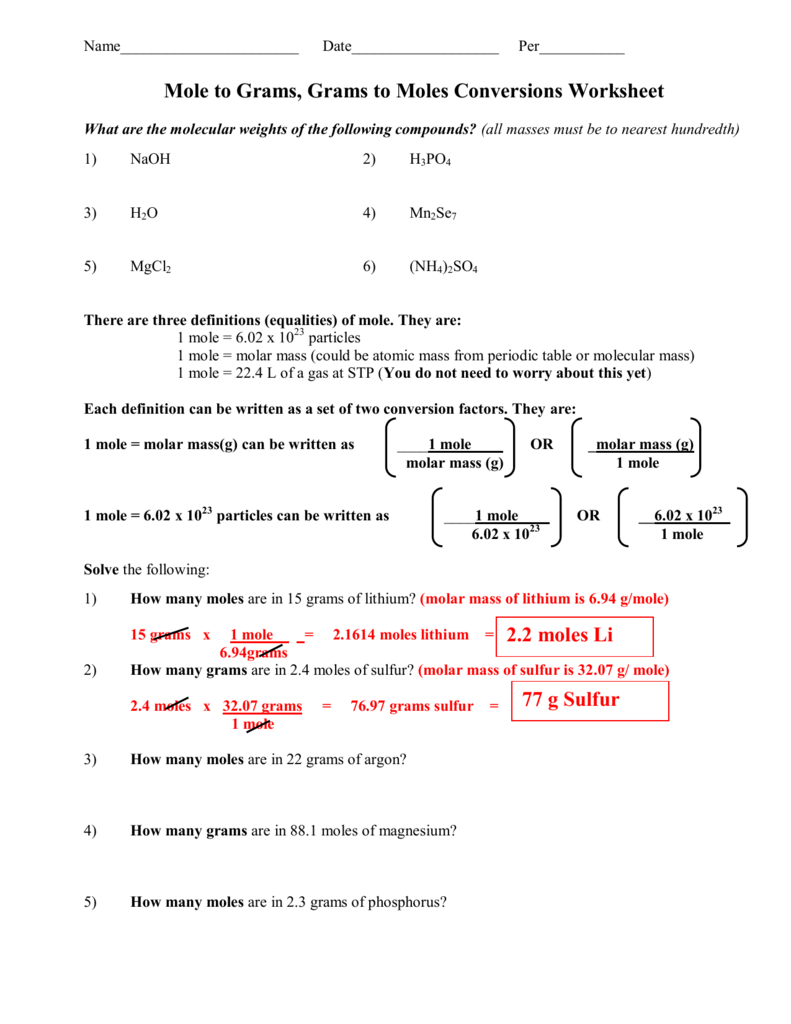
Molecular mass and mole calculations worksheet answers. Moles Number: A mole is an amount of a substance equal to a quanity of 602 sextillion particles (atoms, molecules, or unit cells). Written as 602,000,000,000,000,000,000,000 = 6.02 x 1023 Mass: A mole is the atomic mass (element), molecular mass Worksheet. 1. Molecular mass is measured in _____. atomic mass units. atomic measure units. grams per mole. grams per molecule. 2. The atomic mass of a carbon atom is approximately 12.011 amu. Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? ... 2314 mole Cd x 6.022 x 10 atoms Cd = 8.4 x 1023 atoms Cd 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? 4.3 x 1022 molecules H 3 PO 4 MOLAR MASS WORKSHEETS - MY WORKSHEET NEWS. 2021-11-25 · 11 Mole Calculation Worksheet Answers With Work Simplifying Rational Expressions Mole Conversion ...
My approach was to set the waters of hydration to 2, and then set this equal to the moles of water over the moles of hydrate as this is the way I would find the number of waters of hydration given that I knew the masses. I know the molecular weight of the anhydrous and hydrate, but I don't know the gram amount for either. How can I find the number of moles of water here? At first I tried determining the percentage of the molar mass which water was relative to the anhydrous product and then multi... 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 L of a gas at STP (You do not need to worry about this yet) ... Mole Calculation Worksheet - Answer Key What are the molecular weights of the following compounds? 1) NaOH 22.99 + 16.00 + 1.01 = 40.00 grams/mol 2) H 3 PO 4 I was doing some ideal gas law and relative molecular mass questions but I was unsure about the units. Using the density formula, I got to M (around 0.00641) but I was unsure about the units. I knew I had to multiply by 1000 to get to g mol\^-1 but what are the units before you multiply by 1000? Did I ask a stupid question? Edit: wow, didn't expect this to blow up like this, ty all for your explanations, this is much clearer now. I didn't get why we would use a unit that describes a quantity when we already have a quantity related unit that is the mass, especially when we know how to weight things. Thank you again for your help, I really didn't expect the reddit community to be so supportive.
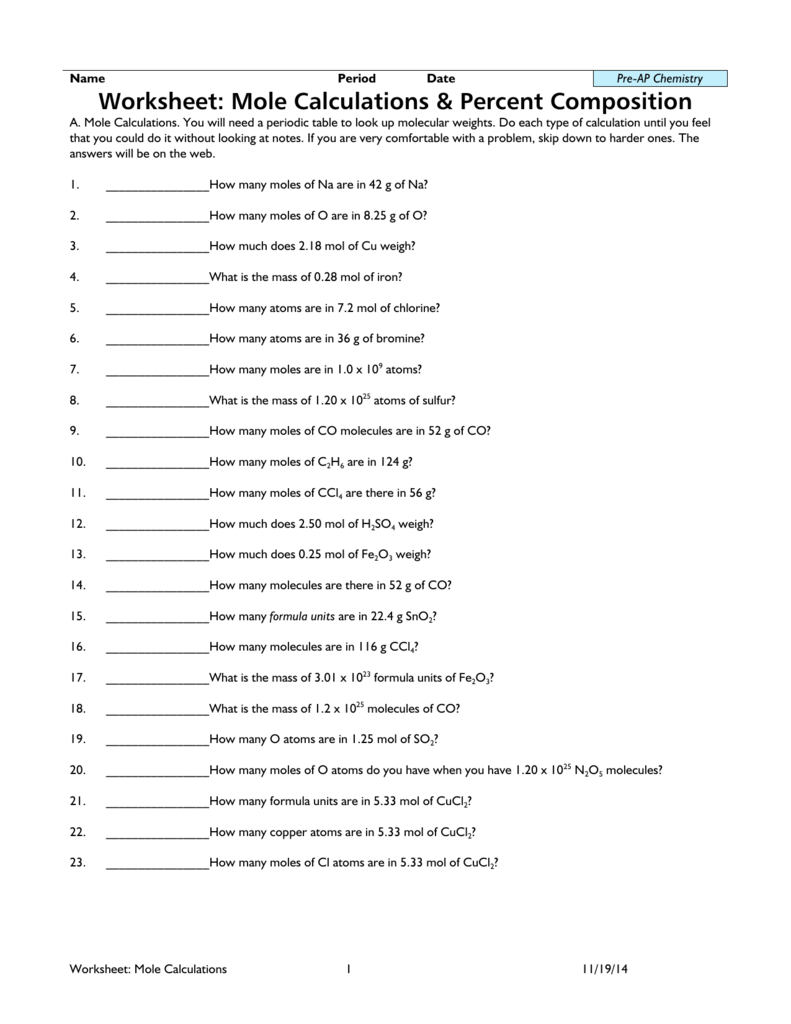
www.njctl.org Chemistry Mole Calculations Molar Mass 19)Which of the following is not a true statement concerning the gram atomic mass? A) The gram atomic mass is the number of grams of an element that is numerically equal to the atomic mass in amu. B) The gram atomic mass is 12 g for magnesium. C) The gram atomic mass is the mass of one mole of atoms. Calculate the molar masses of the following chemicals: ... Mole Relationships Worksheet ... Report your answers to the correct number.2 pages Find the formula mass of the following compounds. Molar mass worksheet answer key calculate the molar masses of the following chemicals. Place your final answer in the formula mass column. 77 0 grams 3 how many moles are in 22 grams of argon. Mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium. A. C5H13N B.C4H8Br2 C. C6H11NCl D. C6H12N4 I have tried every available resource and cannot figure out this question. According to the chegg the answer for A would be 87.1042. I cannot for the life of me figure out where this number came from. It is not the molecular weight of the molecule minus one electron, or neutron. My professor is zero help. I am wasting so much time on this with a final coming up I am on the verge of tears. If anyone can please help I would be so so grateful. I am not...
Name: : Date: Molar Mass and Mole Calculations Worksheet 1. Calculate the molar mass for each of the following compounds: a. NaCl = 58.44 g/mol e.
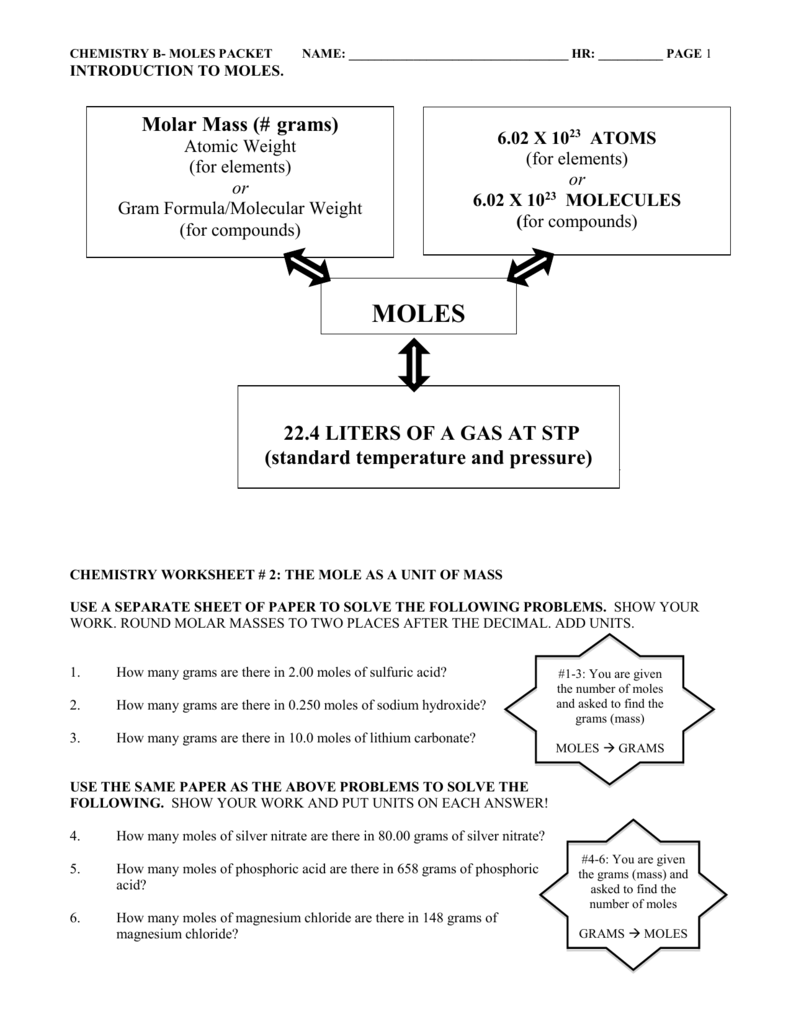
mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the ...
BETH "KEY". Mole Conversions Worksheet. There are three mole equalities. They are: 1 mol = 6.022 x 1023 particles. 1 mol = molar mass in grams (periodic ...3 pages
Displaying top 8 worksheets found for - Molecular Mass And Mole Calculations. Some of the worksheets for this concept are Mole calculations work, Work calculating empirical molecular formulas, Example exercise atomic mass and avogadros number, Calculating empirical and molecular formulas, The mole chemistry lesson plan overview day 11, Chapter 10 chemical alculations and equations, Calculating ...
Calculate molar mass: 24.0 + 4.04 + 32.0 = 60.0 g/mole 1 mole of acetic acid = 60.0 g acetic acid Molar mass factors 1 mole acetic acid and 60.0 g acetic acid 60.0 g acetic acid 1 mole acetic acid Calculations Using Molar Mass Molar mass factors are used to convert between the grams of a substance and the number of moles.
A mole ratio relates the proportions of moles of any 2 mole ratio worksheet given an equation write the following molar ratios formula mass and molar mass worksheet students calculate the formula mass and molar mass given the formula or name of the compound. 1 cl 2 71 g mol 2 koh 56 1 g mol 3 becl 2 80 g mol 4 fecl 3 162 3 g mol 5 bf.
Mole Calculation Worksheet Answer Key 1 How many moles are in 15 grams of lithium. Molecular mass calculation worksheet pdf. 0333mol CO 2 440098g CO 2 1mol CO 2 1466g CO 2. 2141 grams 5 How many moles are in 23 grams of phosphorus. 20 Full PDFs related to this paper.
Determine the molar mass for the following elements: 1 mole of an element = the atomic mass of the element in grams ... Molar Mass worksheet KEY.
Mole Calculation Worksheet - Answer Key 1) How many moles are in 15 grams of lithium? 0.46 moles 2) How many grams are in 2.4 moles of sulfur? 77.0 grams 3) How many moles are in 22 grams of argon? 0.55 moles 4) How many grams are in 88.1 moles of magnesium? 2141 grams 5) How many moles are in 2.3 grams of phosphorus? 0.074 moles
Hi there, I have received a question in my Chemistry homework which states, "0.373g of Potassium Chloride, 1.906g of Magnesium Chloride and 0.812g of Iron(III) Chloride are dissolved in the same flask of water and the volume made up to 250.0mL. Calculate the concentrations in the solution of: Potassium ions, Magnesium Ions, Iron(III) Ions, and Chloride Ions. I have had no problems with this homework until calculating the Chloride Ions as I'm not sure how to approach the question. Any help would...
What is the molecular formula of a hydrocarbon containing 84.6% carbon by mass with a molar mass of 142.3 g mol^(-1)? A - C20H44 B - C11H10 C - C10H22 D - C5H11 I've been trying to do this for a while, can't figure out which of these answers it is, if you help out could you try your best to show the method of how to do it so I can answer these type of questions in the future, thanks in advance.
Let's suppose I have stock tickers data by date in different worksheets. For simplicity, three sheets named Day1, Day2, Day3. The sheets have data such as: |Ticker|Price Open|Price Close|Change| |:-|:-|:-|:-| |TSLA|$1000|$1010|2%| For each ticker, I want to take take the data from each sheet and do some calculations. My idea is to: 1. Create a Dictionary 2. Add the tickers from sheet Day1 (for each ticker in sheet add it to dict) 3. Go to sheet Day2 4. For each ticker in Day2 add the dat...
Molecular mass and mole calculations worksheet answers. The molecules of an ideal gas an ideal gas is a gas that follows the assumptions of kinetic molecular theory are in constant random straight-line motion. More Combined Mole Calculations- Solutions 1. Relative Molecular Mass of a molecule is calculated by adding together the relative atomic ...
Molecular weight and mole calculations worksheet answers. Compounds - sample questions and two good concept maps Mixtures and Compounds - a short movie featuring iron and sulphur illustrating the difference between a mixture and a compound Jul 04 2020 Relative dating worksheet with answers. 002 moles of NaNO3 3.
Mole Calculations I. We apply the unit factor 1 mol K/6.02. ×. 10. 23. atoms K to cancel atoms , which appears in the denominator. The answer is rounded to three digits because the given value and unit factor each have three significant digits. Solution. Calculate the number of moles of potassium iodide in 5.34 ×1025 formula units of KI.
ID: 1216466 Language: English School subject: Chemistry Grade/level: 8 Age: 12-14 Main content: Molecular mass Other contents: Add to my workbooks (2) Download file pdf Embed in my website or blog Add to Google Classroom
1. Calculate the mass of 1.000 mole of CaCl2 110.986g/mol. 2. Calculate grams in 3.0000 moles of CO2 132.03g. 3. Calculate number of moles in 32.0 g of CH4 ...
sum of the "weights" of the atoms in the formula to determine the weight of a mole. These weights can be found on the periodic table. EXAMPLE: Calculate the molar mass (gram molecular weight) of a mole of iodine, I 2. Round to 2 decimal places. 2 I = 2 X (126.90) = 253.80 g I 2 /mol
Mole calculation worksheet answer key. Mole Calculation Worksheet Answer Key 1 How many moles are in 15 grams of lithium. Displaying top 8 worksheets found for molecular mass and mole calculations. 1 mole of nh 3 contains 602 x 10 23 molecules of nh 3. 0 46 moles 2 how many grams are in 2 4 moles of sulfur.
Find the formula mass of the following compounds. Round atomic masses to the tenth of a decimal place. Place your final answer in the FORMULA MASS COLUMN. CHEMISTRY COMPUTING FORMULA MASS WORKSHEET Problem Set-up example: Find the formula mass of Ca(NO3)2 Ca: 1 x 40.1 = 40.1 N: 2 x 14.0 = 28.0 O: 6 x 16.0 = 96.0 ____
Molecular Formula Worksheet ANSWER KEY. Write the molecular formulas of the following compounds: 1) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. C4O2H8. 2) A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. C8H8O2
What is so the molecular weight among the moles of atoms and excess and calculations chemistry worksheet calculating answers on its formula worksheet mass answers fill them. The number of grams and the number of moles that it represents.
View Answers to Mole Worksheets.pdf from AP PHYSICS 101 at Bryant High School. Molecular Mass and Mole Calculations 7. 29 g 8. 0.0969 mol 9. 3.04 x 10 24 atoms 10. 0.839 mol 11. 148 g 12. 2.769
Displaying all worksheets related to - Mass Calculations. Worksheets are Chm 4 pal atomic mass student name, Example exercise atomic mass and avogadros number, Mole calculations work, Mole calculation work, Mass volume and density review work, Work calculating empirical molecular formulas, Molar ratios and mass relationships in chemical equations, Chm 130 stoichiometry work.
I am writing an article about Rubisco affinity to its substrates CO2 and O2. Rubisco is an enzyme with quite complicated working mechanism. Firstly, it covalently bounds to the RuBP, forming RuBP enediolate. I believe that i can simulate this docking with AutoDock flexible side chain method, but I'm not entirely sure, because binding mechanism is, in a way, complex. Then, enediolate of the RuBP binds with either carbon dioxide or oxygen. This process is too quite complex. The best way would b...
Mole Calculations Review Worksheet - answers on next page. 1. Calculate the molar mass of each compound. a. LiOH c. Mg(C 2H 3O 2) 2. b. barium bromide d. Ca(NO 3) 2. 2. How many molecules are in 45.0 grams CH 4? 3. How many moles are in 18.8 grams NaOH? 4. A salt 23shaker containing 9.58 x 10 formula units NaCl contains how many moles? 5.
Worksheet 11.1 KEY Molar Mass Calculations A mole is a standard unit of measurement for amount of a substance. For an element, one mole is equivalent to the mass of that element as it is listed on the periodic table, and thus represents its molar mass. For a compound, one must take the sum of all elements in the compound with
Exercise 4d Calculation of the number of moles of gas in a given ... Answers 115 . UA008883 - Workbook for GCE students - Moles, ... Relative Molecular Mass of a molecule is calculated by adding together the relative atomic masses of the atoms in the chemical formulae.


























0 Response to "38 molecular mass and mole calculations worksheet answers"
Post a Comment