42 empirical and molecular formulas worksheet
Percent Composition and Molecular Formula Worksheet -. ...and Molecular Formula Worksheet1.What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen?2.If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula?3.What's the empirical formula of a molecule... Empirical and Molecular Formulas Worksheet Some of the worksheets displayed are Empirical and molecular formula work, Empirical and molecular formulas work, Chemistry, , Empirical formula work, Work 8 empirical formulas h o n o 4i, Compound names and formulas work three, Chemical formula writing work two.
Calculate Empirical and Molecular Formulas Empirical and Molecular Formula Key Takeaways. The empirical formula gives the smallest whole number ratio between elements in a compound. For some molecules, the empirical and molecular formulas are the same. Usually, the molecular formula is a multiple of the empirical formula.
Empirical and molecular formulas worksheet
3.2 Determining Empirical and Molecular Formulas - Chemistry Determine the molecular formula of a compound. In the previous section, we discussed the relationship between the bulk mass of a substance and the number of atoms or Determining Percent Composition from a Molecular Formula Aspirin is a compound with the molecular formula C9H8O4. › molecular-formula-practiceMolecular Formula Practice Test Questions - ThoughtCo Aug 01, 2019 · The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds. A periodic table will be required to complete this test. Answers appear after the final question. Empirical and Molecular Formulas Worksheets | PDF | Mole (Unit) Empirical and Molecular Formulas The empirical formula of a compound gives the simplest whole number ratio of different. Empirical formulas may be easily determined from experimental data. Usually you must first determine how many grams of each type of atom are in the compound.
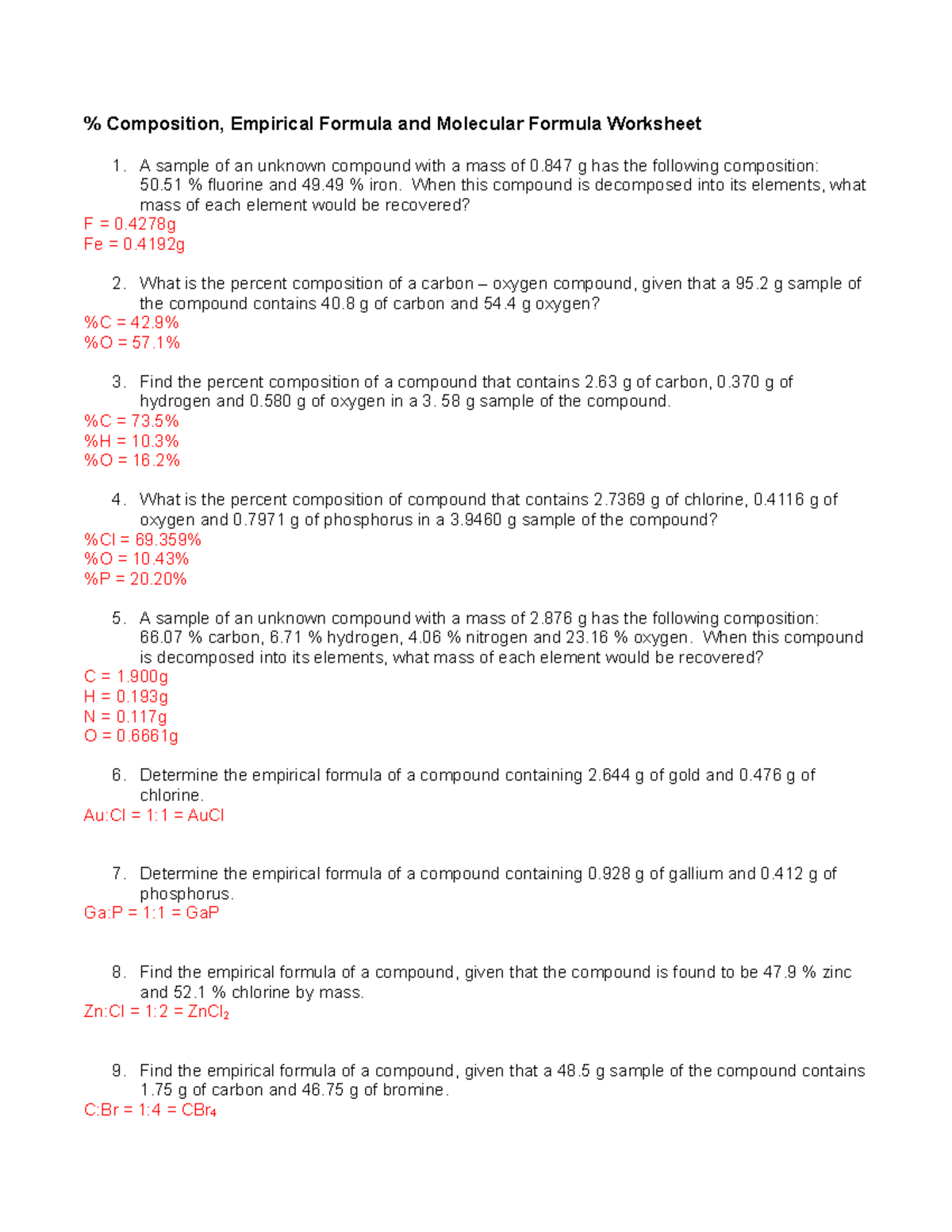
Empirical and molecular formulas worksheet. Empirical and Molecular Formulas | Interactive Worksheet by... Empirical and Molecular Formulas Worksheet. April 18, 2020. Impact. Ibuprofen, a common headache remedy, has an empirical formula of C7H9O. What could be the possible molecular formula? PDF Chemistry Worksheet | Empirical Formulas Empirical and Molecular Formulas. The purposes of this worksheet are for you to practice using experimental data to determine the empirical formula of a compound, and given additional information to determine the actual molecular formula of the compound. Percent Composition and Molecular Formula Worksheet 3 Molecular Formula Worksheet ANSWER KEY Write the molecular formulas of the following compounds: 1) A compound with an Li 2 CO 3 (In this case, the molecular and empirical formulas are the same, a frequent occurrence for inorganic compounds) 9. The percentage composition of... Empirical and Molecular Formulas by Drew Pindelski Empirical and Molecular Formulas. 56. Learn about Prezi. this compound is? Empirical Formula: The simplest whole number ratio of elements in a formula. 1) Work on a 100 g basis. 2) Convert to Moles.
Empirical and Molecular formula worksheet Mole concepy online worksheet for Pre-Uni. Empirical and Molecular formula Example d in the lecture notes. ID: 2238973 Language: English School subject: Chemistry Grade/level: Pre-Uni Age: 18+ Main content: Mole concepy Other contents PDF Microsoft Word - Answers to Worksheet 8.doc Answers to Worksheet #8 Empirical Formulas To calculate empirical formulas, follow the steps outlined below: (assume percentages given in the problems are grams) Step 1: convert to moles Step 2: divide each by the lowest number of moles Step 3: (only if necessary) multiply all by the same factor in order to obtain whole numbers. . 3.2: Determining Empirical and Molecular Formulas - Chemistry... Determining the Empirical Formula of Penicillin. Combustion Analysis. From Empirical Formula to Molecular Formula. In 1928, Alexander Fleming, a young microbiologist at the University of London, was working with a common bacterium that causes boils and other infections such as blood poisoning. Determining Empirical Formulas Worksheets - Lesson Worksheets Worksheets are Empirical and molecular formulas work, Empirical and molecular formula work, , Empirical formula work, Work 8 empirical formulas h o n o 4i, Percent composition and molecular formula work, , Determining molecular formulas true formulas. Click on pop-out icon or print icon...
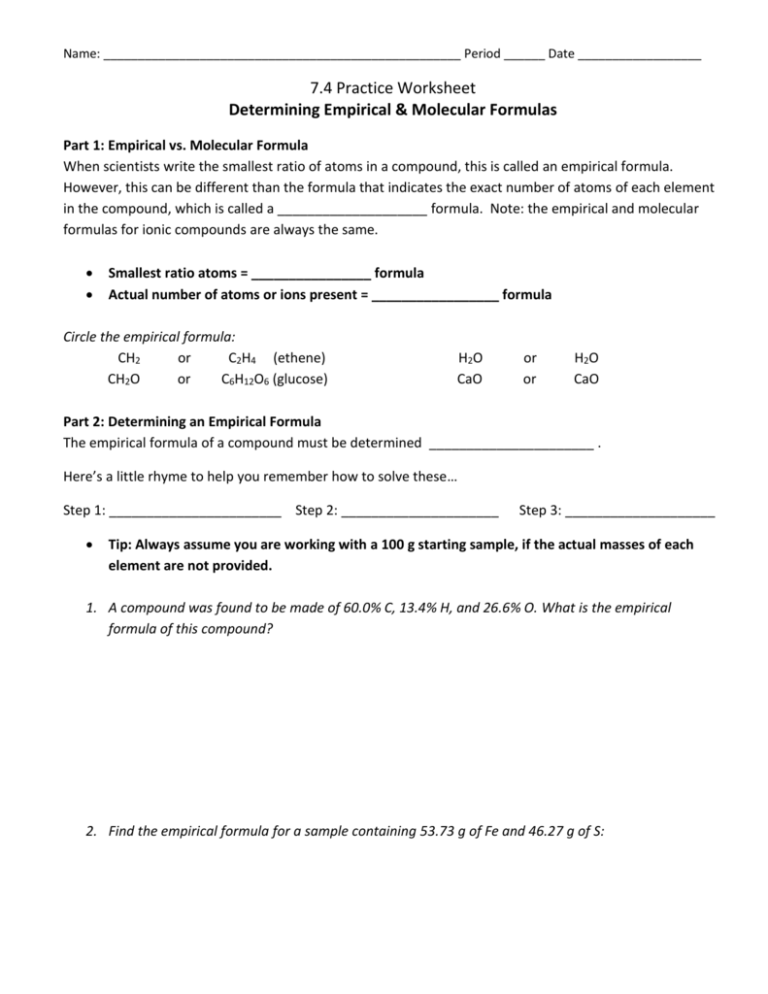
PDF Empirical Formula Worksheet 1 Empirical Formulas Worksheet, #1. Pd. Directions: Find the empirical formula and name for each of the following. 9. A compound is 19.3% Na, 26.9% S, and 53.8% O. Its formula mass is 238 g. What is its molecular formula? 10. An experiment uses a catalyst that is 23.3% Co, 25.3% Mo, and 51.4... PDF Empirical formula worksheet Empirical formula worksheet. Problems that involve percentages, mass or moles of elements: Follow the poem. Show your work. One last reminder: the empirical formula and the molecular formula are related by a whole number ratio. PDF Percent Composition | Empirical vs. Molecular Empirical formula vs. MOLECULAR FORMULA uEmpirical Formula: the simplest. whole number ratio of atoms in a compound. uMolecular Formula: shows how many atoms of each element are present in a molecule or compound. Empirical vs. Molecular. Empirical vs Molecular Formula | Science Notes and Projects The empirical and molecular formulas are two types of chemical formulas that tell you the ratios or proportions of elements in a compound. Here are examples of empirical and molecular formulas and worked problems showing how to find these formulas from mass percentages and molecular...
Empirical and Molecular Formula Worksheet Show work on a separate sheet of paper. Write the empirical formula for the following compounds. 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. What is the molecular formula of this compound?
Empirical and Molecular Formula Worksheet - Learny Kids Worksheets are Empirical and molecular formula work, , Emp... Displaying top 8 worksheets found for - Emperical Formulas. Some of the worksheets for this concept are Empirical and molecular formula work, , Empirical formula work, Work 8 empirical formulas h o n o 4i...
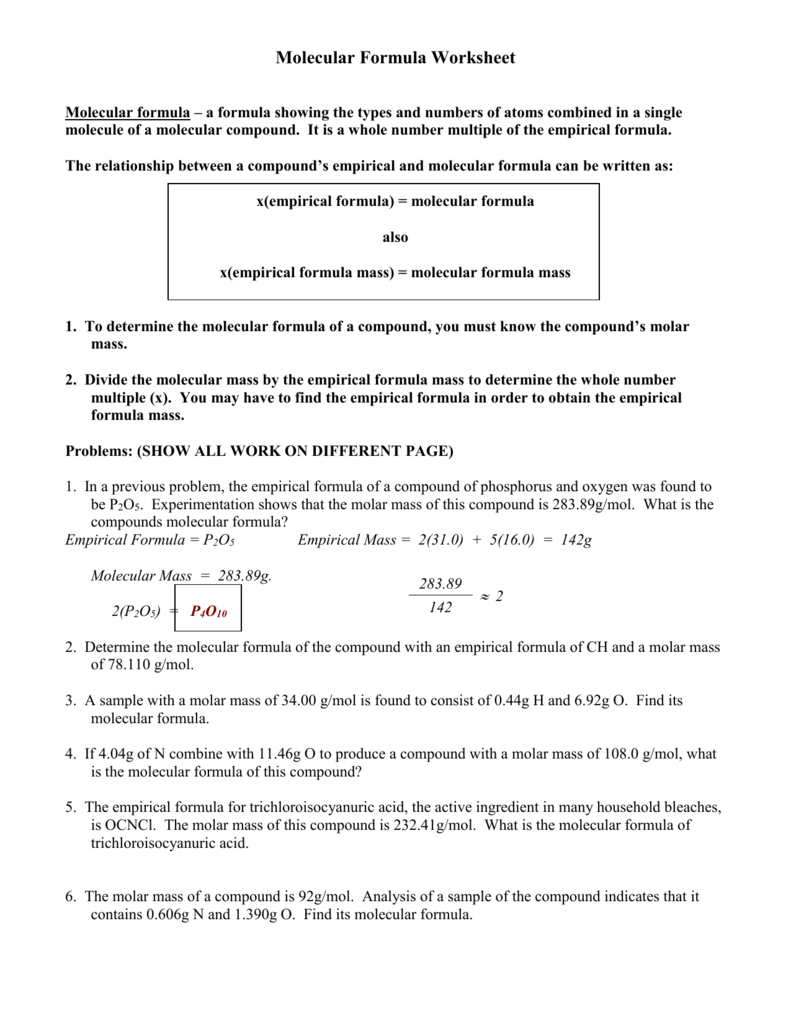
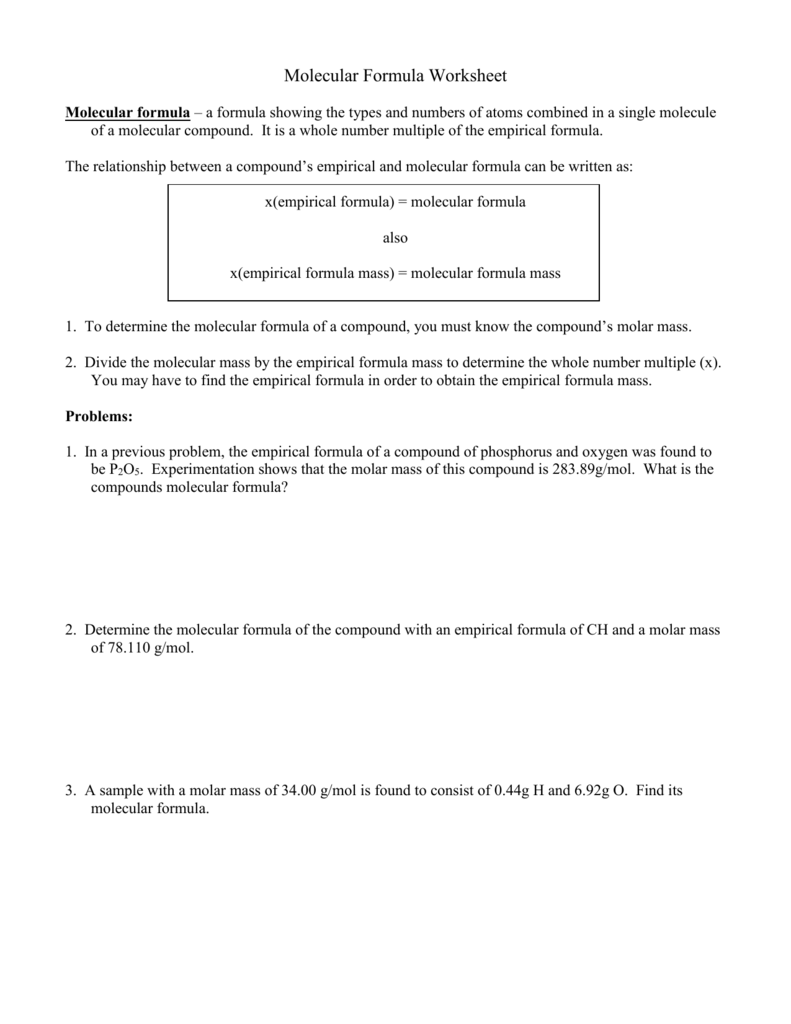
DOC Molecular Formula Worksheet Molecular formula - a formula showing the types and numbers of atoms combined in a single molecule of a molecular compound. It is a whole number multiple of the empirical formula. The relationship between a compound's empirical and molecular formula can be written as
PDF Empirical and Molecular Formulas Worksheet Empirical Formulas Worksheet. Objectives: • be able to calculate empirical formulas. Empirical formula • Expresses the simplest ratios of atoms in a compound • Written with the smallest whole-number subscripts.
Empirical & Molecular Formulas Flashcards | Quizlet Start studying Empirical & Molecular Formulas. Learn vocabulary, terms and more with flashcards, games and other study tools. Glycerol has a molar mass of 92.09g/mol. Its percent composition is: 39.12% C, 8.75% H, and 51.12% O. What is the molecular formula for glycerol?
PDF Microsoft Word - Empirical and Molecular Formula Worksheet.docx Empirical and Molecular Formula Worksheet. 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula?
Worksheets - Empirical Formula 1. Determine the empirical formula from the molecular formula d) A compound analyzes as 79.08 % C; 5.54 % H and 15.38 % N. What is the molecular formula if the molar mass is 273.36 g/ mol?
opentextbc.ca › chapter › 2-4-chemical-formulas2.4 Chemical Formulas – Chemistry Write the molecular and empirical formulas of the following compounds: (a) (b) (c) (d) Determine the empirical formulas for the following compounds: (a) caffeine, C 8 H 10 N 4 O 2 (b) fructose, C 12 H 22 O 11 (c) hydrogen peroxide, H 2 O 2 (d) glucose, C 6 H 12 O 6 (e) ascorbic acid (vitamin C), C 6 H 8 O 6. Determine the empirical formulas for ...
chem.libretexts.org › Courses › Oregon_Institute_of4.3: Empirical and Molecular Formulas ... - Chemistry LibreTexts Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)
Determining Empirical and Molecular Formulas | Chemistry I The empirical formula for this compound is thus CH 2 . This may or not be the compound's molecular formula as well; however, we would need additional information to make that determination Recall that empirical formulas are symbols representing the relative numbers of a compound's elements.
› test-prep › mcatStoichiometry questions (practice) - Khan Academy Empirical formula from mass composition edited. Molecular and empirical formulas. The mole and Avogadro's number. Stoichiometry example problem 1. Stoichiometry.
5.4 Determining Empirical and Molecular Formulas - CHEM 1114... The empirical formula is the simplest formula of a compound. It is the smallest whole number ratio of atoms, but does not necessarily represent the arrangement of atoms in the actual molecule. For example: a molecule of hydrogen peroxide is made up of two atoms of O and two atoms of H bonded...
empirical and molecular formulas worksheet - Bing Molecular Formulas To calculate molecular formulas, follow the steps outlined below: Step 1: calculate empirical formula (see above) Step 2: divide EMPIRICAL AND MOLECULAR FORMULA WORKSHEET An oxide of chromium is found to have the following % composition: 68.4 % Cr and...
PDF Empirical and Molecular Formula Worksheet Write the empirical formula for the following compounds. 7) A compound with an empirical formula of C4H4O and a molar mass of 136 grams. per mole. What is the molecular formula of this compound?
› userfiles › 1002Percent Composition and Molecular Formula Worksheet Molecular Formula Worksheet ANSWER KEY Write the molecular formulas of the following compounds: 1) A compound with an empirical formula of C 2 OH 4 and a molar mass of 88 grams per mole. C 4 O 2 H 8 2) A compound with an empirical formula of C 4 H 4 O and a molar mass of 136 grams per mole. C 8 H 8 O 2
Empirical And Molecular Formulas Worksheet , Jobs EcityWorks Empirical and Molecular Formula Worksheet. Show work on a separate sheet of paper. Write the empirical formula for the following Worksheet: Calculating Empirical & Molecular Formulas 1. The empirical formula for the compound having the formula H2C2O4 is [A] C2H2 [B]...
Empirical and Molecular Formulas Worksheets | PDF | Mole (Unit) Empirical and Molecular Formulas The empirical formula of a compound gives the simplest whole number ratio of different. Empirical formulas may be easily determined from experimental data. Usually you must first determine how many grams of each type of atom are in the compound.
› molecular-formula-practiceMolecular Formula Practice Test Questions - ThoughtCo Aug 01, 2019 · The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds. A periodic table will be required to complete this test. Answers appear after the final question.
3.2 Determining Empirical and Molecular Formulas - Chemistry Determine the molecular formula of a compound. In the previous section, we discussed the relationship between the bulk mass of a substance and the number of atoms or Determining Percent Composition from a Molecular Formula Aspirin is a compound with the molecular formula C9H8O4.

.JPG)

























0 Response to "42 empirical and molecular formulas worksheet"
Post a Comment