43 section 10.3 percent composition and chemical formulas worksheet answers
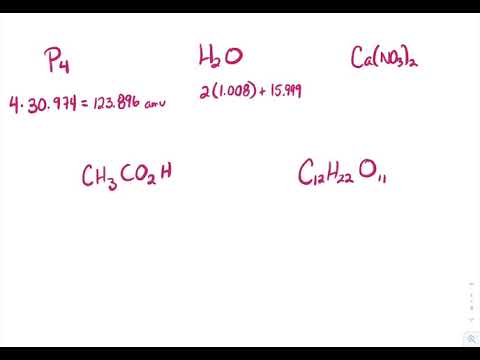
PDF Chapter 10 Chemical Quantities Worksheet Answers Chapter 10 Chemical Quantities Worksheet Section 10.3 - Percent Composition and Chemical Formulas. The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100. mass of compound 10.3 Percent Composition and Chemical Formulas 10.3 Percent Composition and Chemical Formulas 15 > Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.. You can also calculate the percent
Section 103 Percent Composition And Chemical Formulas ... 103 percent composition and chemical formulas sa j use this completion exercise to check your kllowledge of the terms and your understanding of the concepts introduced in section. The number of grams of the element divided by the mass in. 10 3 Percent Composition Chemical Formulas Answer Key Answers. Grade 11 University Chemistry.

Section 10.3 percent composition and chemical formulas worksheet answers
PDF Mirolli Chemistry - Home 2) Find the percent composition of calcium phosphate. 310-3 3) Find the percent composition of magnesium chromate. Croq - 4) A sample of iron (Ill) oxide has a mass of2.9g. After analysis, it was found to contain 1.7g of iron and I .2g of oxygen. Find the percent composition of this compound. 5) Determine the empirical formula of a compound ... PDF Useful Advice - Home Created Date: 2/13/2013 10:31:21 AM PPTX Chapter 10 - Chemical Quantities - Weebly Section 10.3 - Percent Composition and Chemical Formulas. The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100. mass of compound
Section 10.3 percent composition and chemical formulas worksheet answers. Chapter 10.3 Percent Composition and Chemical Formulas ... Start studying Chapter 10.3 Percent Composition and Chemical Formulas. Learn vocabulary, terms, and more with flashcards, games, and other study tools. 10.2 Mole-Mass and Mole- Volume Relationships 10.3 Percent Composition and Chemical Formulas. 10.2 Mole-Mass and Mole- ... formula of the substance, in this case O 2 or O. The Mole-Mass ... • The answer should be close to 1000 g. • The answer has been rounded to the correct number of significant figures. Sample Problem 10.5 PDF Livingston Public Schools / LPS Homepage Subject: Image Created Date: 1/3/2013 3:17:41 PM PDF Ch 10 Study Guide TE - Mr. McKnight Clawson High School Section 10.4 Empirical and Molecular Formulas 1. The percent composition of a compound is the percent by mass of each of the elements in a compound. 2. Divide the mass of each element in the sample by the mass of the sample. Then multiply each quotient by 100. 3. c 4. c 5. b 6. b 7. c 8. c 9. d 10. 7.89 g K 1 mol K/39.10 g K 0.202 mol K 2.42 g C
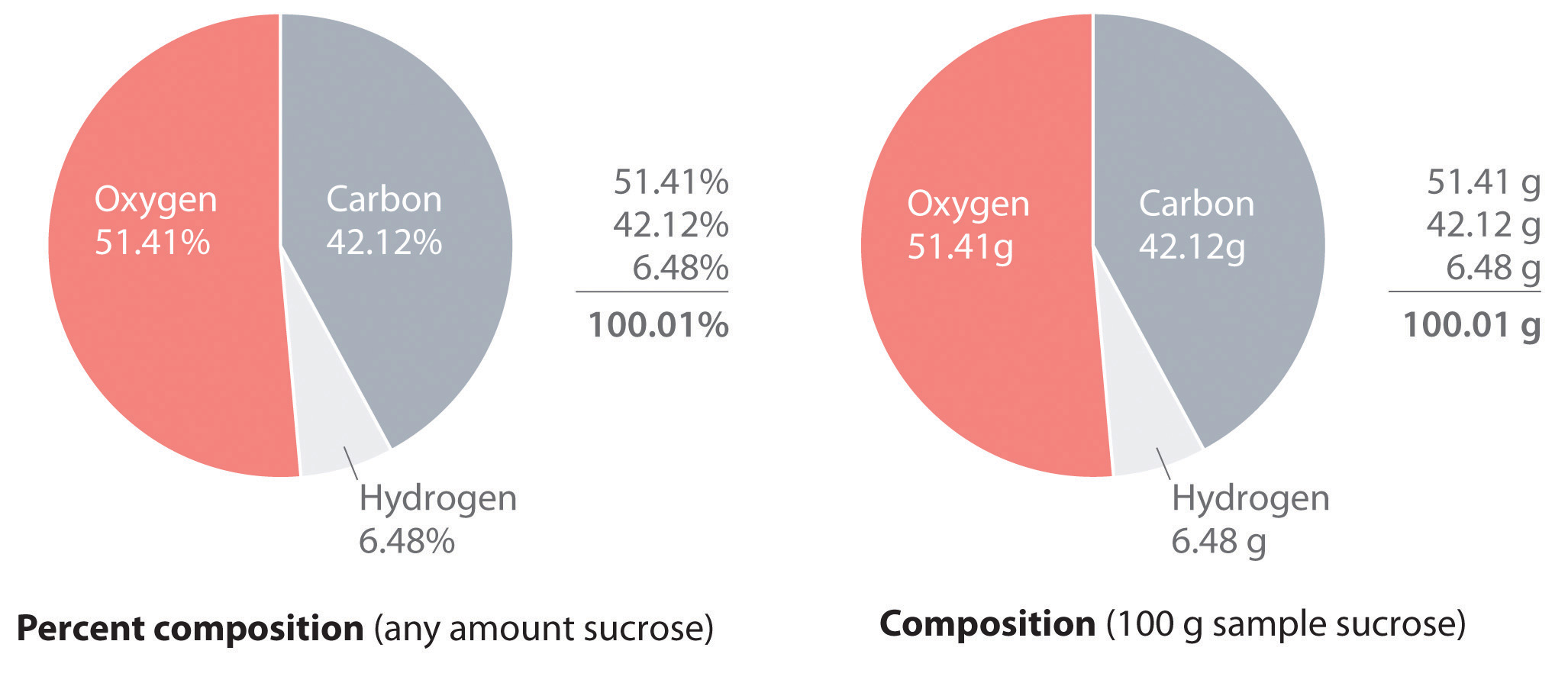
PDF Skills Worksheet Concept Review Holt Chemistry 1 The Mole and Chemical Composition Section: Avogadro's Number and Molar Conversions Solve the following problems, and write your answer in the space provided. 1. Determine the number of atoms present in 4.00 mol of aluminum. 2. Determine the number of atoms present in 1.55 mol of sodium. 3. PDF 10.3 Percent Composition and Chemical Formulas 10.3 Percent Composition and Chemical Formulas A molecular formula of a compound is a whole-number multiple of its empirical formula. Lesson Summary Percent Composition of a Compound Percent composition is the percent by mass of each element in a compound. To find the percent by mass of an element in a compound, use the formula: massof element 10.3 Percent Composition & Chemical Formulas Answer Key ... 10.3 Percent Composition & Chemical Formulas Answer Key/Answers - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Here are the answers for chemistry 10.3 Percent Composition & Chemical Formulas PDF SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287-296) Chapter 10 Chemical Quantities95 SECTION 10.3 PERCENT COMPOSITION AND CHEMICAL FORMULAS (pages 305-312) This section explains how to calculate percent composition from chemical formulas or experimental data, and how to derive empirical and molecular formulas. Percent Composition of a Compound (pages 305-308) 1.
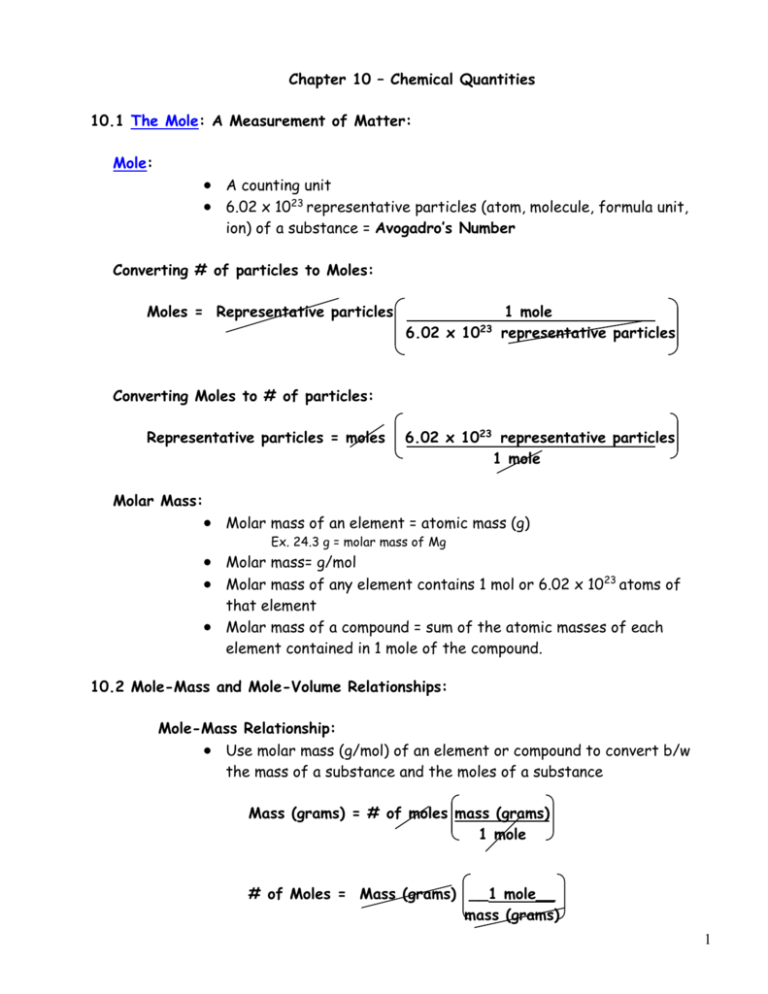
PDF CH.10 THE MOLE - Chemistry Ch.10 - The Moles Mole: (working definition) the mass of a compound that is the same number of grams as the compound's formula or molecular mass in amu. Mole: (formal definition) the amount of matter that contains as many objects (atoms, molecules, etc.) as the number of atoms in exactly 12 g of 12C. Avogadro's constant: 1 mole = 6.02 x 1023 atoms, molecules, etc. Chapter 10 - Chemical Quantities - 10.3 Percent ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 48 Answer 313.6 Work Step by Step H= 124 g/mol Ca (CHO) = 158.1658 H= 124 x 4 = 496 496/158.1658 x100 =313.6 Update this answer! You can help us out by revising, improving and updating this answer. Update this answer 10.1 The Mole: A Measure- ment of Matter - Pittsfield 10.3 Percent Composition and Chemical Formulas. 10.1 The Mole: A Measure- ... so the answer has the unit kg. Sample Problem 10.1 2 Calculate Solve for the unknown. 90 apples × × = 15 kg apples ... Chemical formula Representative particles in 1.00 mol Copper Atom Cu 6.02 × 1023 Quia Created Date: 2/3/2014 8:17:21 AM
Chapter 10 - Chemical Quantities - 10.3 Percent ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 45 Answer The molecular formula of a compound is either the same as its experimentally determined empirical formula, or it is a simple whole-number multiple of its empirical formula.
PDF Chemistry: Matter and Change Percent Composition (cont.) • The percent by mass of each element in a compound is the percent composition of a compound. • Percent composition of a compound can also be determined from its chemical formula. SECTION 10.4 Empirical and Molecular Formulas
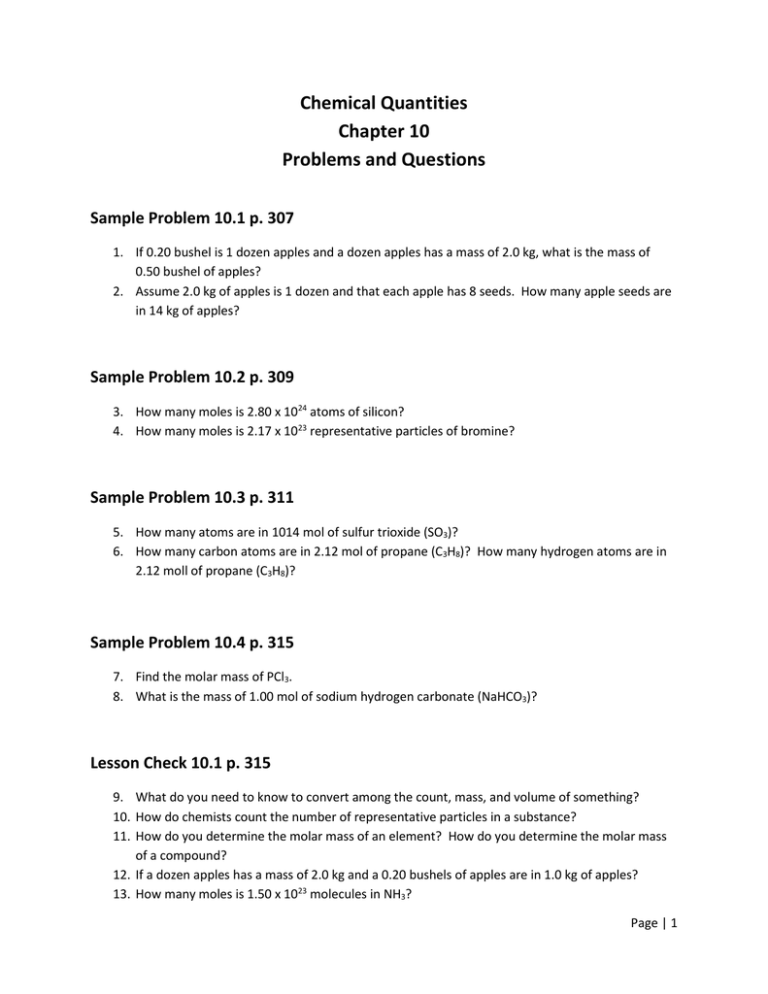
PDF Chapter 10 Chemical Quantities Practice Problems Answers Section 10.3 - Percent Composition and Chemical Formulas. The percent by mass ... Chapter 10 - Chemical Quantities Access Free Chapter 10 Chemical Quantities Answers Chapter 10 Chemical Quantities Answers If you ally habit such a referred chapter 10 chemical quantities answers book that will present you worth, get the extremely best seller ...
PDF 10.3 Percent Composition and Chemical Formulas 10 Section 10.3 Percent Composition and Chemical Formulas 305 10.3 Percent Composition and Chemical Formulas Is your shirt made of 100 percent cotton or wool, or is the fabric a combination of two or more fibers? A tag sewed into the seam of the shirt usually tells you what fibers were used to make the cloth and the percent of each.
PDF 103 Percent Composition And Chemical Formulas Worksheet ... 10.3 Percent Composition and Chemical Formulas 10.3 Percent Composition and Chemical Formulas A molecular formula of a compound is a whole-number multiple of its empirical formula. Lesson Summary Percent Composition of a Compound Percent composition is the percent by mass of each element in a compound.
PPTX Chapter 10 - Chemical Quantities Section 10.3 - Percent Composition and Chemical Formulas The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100 mass of compound Sample Problem
PDF 103 Percent Composition And Chemical Formulas Worksheet ... Composition And Chemical Formulas Worksheet AnswersPercent Composition from a Chemical Formula. The percent composition of a compound can also be determined from the formula of the compound. The subscripts in the formula are first used to calculate the mass of each element in one mole of the compound. That is divided by the molar 103 Percent ...
10.3-Percent Composition and Chemical Formulas Flashcards ... Start studying 10.3-Percent Composition and Chemical Formulas. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
PDF Interpreting Graphics Answers Chemistry Section 14 3 answer key biology 11, chem te ch05 henry county school district, interpreting graphics taxonomy the biology corner, interpreting graphics tvgreen com, 3 interpreting graphs name date class interpreting, 11 interpreting graphics lps, chapter 11 small scale lab, 10 3 percent composition and chemical formulas 10, 14 3 ideal gases grandview
Welcome to CK-12 Foundation | CK-12 Foundation Dichlorine heptoxide (Cl 2 O 7) is a highly reactive compound used in some synthesis reactions. Calculate the percent composition of dichlorine heptoxide. Step 1: List the known quantities and plan the problem. Known. mass of Cl in 1 mol Cl 2 O 7 = 70.90 g. mass of O in 1 mol Cl 2 O 7 = 112.00 g.
PPTX Chapter 10 - Chemical Quantities - Weebly Section 10.3 - Percent Composition and Chemical Formulas. The percent by mass (percent composition) of an element in a compound is the number of grams of the element divided by the mass in grams of the compound multiplied by 100%. % mass of element = mass of element x 100. mass of compound
PDF Useful Advice - Home Created Date: 2/13/2013 10:31:21 AM
PDF Mirolli Chemistry - Home 2) Find the percent composition of calcium phosphate. 310-3 3) Find the percent composition of magnesium chromate. Croq - 4) A sample of iron (Ill) oxide has a mass of2.9g. After analysis, it was found to contain 1.7g of iron and I .2g of oxygen. Find the percent composition of this compound. 5) Determine the empirical formula of a compound ...





























0 Response to "43 section 10.3 percent composition and chemical formulas worksheet answers"
Post a Comment