39 5.3 physics and the quantum mechanical model worksheet answers
5 3 Physics And The Quantum Mechanical Model Section Review Answer Key File Type PDF 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key pro5vps.pnp.gov.ph challenging, its true quality is the sheer simplicity of fundamental physical theories--theories and concepts that can enrich your view of the world around you. COLLEGE PHYSICS, Tenth 5.3 Review - Name Date Class PHYSICS AND THE QUANTUM MECHANICAL MODEL ... name date class physics and the quantum mechanical model section review objectives describe the relationship between the wavelength and frequency of light explain how the frequencies of light are related to changes in electron energies distinguish between quantum mechanics and classical mechanics identify the cause of the atomic emission …
Electrons In Atoms Assessment Answers Prentice Hall Pdf Free Download Chapter 5 Electrons In Atoms Worksheet Answers Worksheet Answersworksheet Answers That We Will Utterly Offer. It Is Not In The Region Of The Costs. ... Arrangement Of Electrons In Atoms Answer Key October 10th, 2019 - 5 Study Guide Key Concepts WithChemASAP 5 1 Models Of 5 3 Physics And The Quantum Mechanical Model 3 D 7th, 2022. Related Book ...
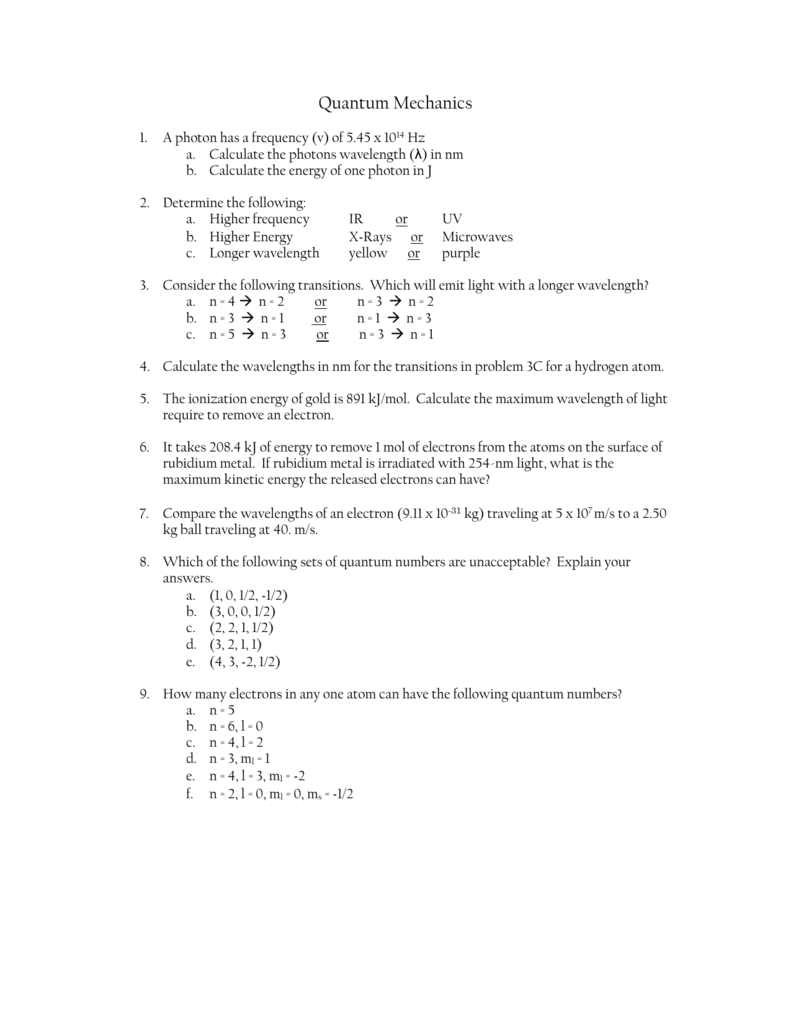
5.3 physics and the quantum mechanical model worksheet answers
PDF A Level Physics Particles Answers AQA - MME A Level Physics Particles (Answers) Name: Total Marks: /30 . 1.This question explores the fundamental forces that are invoked in the standard model and that act at the quantum level. Total for Question 1: 10 (a) State the three fundamental interactions that are described by the Standard Model. ... Solution: In classical mechanics, we would ... PPTX Ch5 Modern Atomic Theory - Henry County Schools / Overview The Quantum Mechanical Model. Rutherford's and Bohr's model focused on describing the path of the electron around the nucleus like a particle (like a small baseball). Austrian physicist . Erwin Schrödinger (1887-1961) treated the electron as a wave. The modern description of the electrons in atoms, the . quantum mechanical model Electrons In Atoms Practice Problems Answer Key Pdf Download Chapter 13 Electrons In Atoms Practice Problems Answers (chapter 2.5 And 2.6) 1. No One Resonance Forms Accurately Depicts The Structure Of The Molecule. The Real Structure Is A Composite Or Hybrid Of All Resonance Forms 2. Resonance Forms Differ Only By The Placement Of π- Or Non-bonding Electrons.
5.3 physics and the quantum mechanical model worksheet answers. 5.3 Atomic Emission Spectra And The Quantum Mechanical Model 5.3 Atomic Emission Spectra And The Quantum Mechanical Model STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by Anna_Gilmore28 Terms in this set (35) The Amplitude Of a Wave The waves height from zero to the crest The Wavelength Is the distance between the crests Frequency 5.3 physics and the Quantum Mechanical Model - Quizlet Start studying 5.3 physics and the Quantum Mechanical Model. Learn vocabulary, terms, and more with flashcards, games, and other study tools. PDF chem TE ch05 - Henry County Schools Section 5.3 Physics and the Quantum Mechanical Model139 The product of frequency and wavelength always equals a constant (c), the speed of light: cλν The wavelength and frequency of light are inversely proportional to each other. As the wavelength of light increases, for example, the frequency decreases. Sample Problems and Solutions - Physics Classroom Determine the acceleration of the car and the distance traveled. See Answer See solution below. A feather is dropped on the moon from a height of 1.40 meters. The acceleration of gravity on the moon is 1.67 m/s 2. Determine the time for the feather to fall to the surface of the moon. See Answer See solution below.
Quantum Physics | Physics Quiz - Quizizz 30 seconds. Report an issue. Q. Quantum physics describes: answer choices. Nature at the smallest scales of energy levels of atoms and macroatomic particles. Nature at the biggest scales of energy levels of atoms and subatomic particles. Nature at the smallest scales of saiyajin levels of atoms and subatomic particles. 5.3 notes - Chapter 5.3 Atomic Emission Spectra and the Quantum ... • In the visible spectrum, red light has the longest wavelength and the lowest frequency. Chapter 5.3-1 The electromagnetic spectrum consists of radiation over a broad range of wavelengths. When atoms absorb energy, their electrons move to higher energy levels. These electrons lose energy by emitting light when they return to lower energy levels. PDF Weebly multiples of —hv, Whv, and so on. energy The is the phenomenon in which electrons are emitted from a metal's surface Electromagnetic radiation is a kind of (1) when light of a certain frequency shines on it. Wave as it travels through space. (3) Light is one type of a. quantum b. PDF Solved Problems in Classical Mechanics on undergraduate and graduate courses in classical mechanics, and the variety of excellent textbooks on the subject. It has, furthermore, been recognized that training in this and related branches of physics is useful also to students whose careers will take them outside physics. It seems that here the problem-solvingabilities that physics
Section 5.2 Quantum Theory and the Atom Worksheet - Quizlet Bohr- only hydrogen atom, ground state, single electron moves in circular orbits around nucleus assigned quantum number, n, to each orbit; Quantum Machanical- treats electrons as waves, no attempt to describe electron's path, atomic orbital. Atomic orbitals ___ have an exactly defiend size. do not Each orbital may contain at most ___ electrons. two Work and Energy Review - with Answers #3 - Physics Classroom Approximate the work required lift a 2.5-kg object to a height of 6.0 meters. PSYW. Answer: ~150 J. The work done upon an object is found with the equation. W = F*d*cos (Theta) In this case, the d=6.0 m; the F=24.5 N (it takes 24.5 N of force to lift a 2.5-kg object; that's the weight of the object), and the angle between F and d (Theta) is 0 ... PDF Livingston Public Schools / LPS Homepage Use each of the terms below to complete the statements. 1. The lowest allowable energy state of an atom is called its 2. Bohr's model of the atom predicted the hydrogen's atomic emission spectrum. 3. According to Bohr's atomic model, the smaller an electron's orbit, the the atom's energy level. 4. 5.3: Physics and the Quantum Mechanical Model Flashcards - Quizlet Start studying 5.3: Physics and the Quantum Mechanical Model. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
5.3 Physics and the Quantum Mechanical Model - Quizlet 5.3 Physics and the Quantum Mechanical Model STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Amplitude Click card to see definition 👆 The waves height from zero to crest Click again to see term 👆 1/11 Created by jblake16 Terms in this set (11) Amplitude The waves height from zero to crest Wavelength The distance between the crests
PDF 5 Electrons In Atoms Key Answers energy 1s has one electron then a second electron of opposite spin the next orbital to fill is 2s it also has one electron then a second electron of opposite spin''chapter 5 electrons in atoms december 26th, 2019 - ation shown in figure 5 5 you should note that energy increases with increasing frequency thus looking back atfigure 5 3 the violet …
PPTX Chapter 5 - Electrons in Atoms - Henry County Schools The quantum mechanical model stills has energy levels, but the exact path or orbit of the electron is unknown. ... Section 5.3 - Physics and the Quantum Mechanical Model. There are 4 properties of a wave that you need to be able to identify. The crest is the highest point of a wave.
PDF A Level Quantum Physics Answers OCR - MME matter and space. For example, the photon model accurately explains what happens when electromag-netic radiation reaches the surface of a solid but to explain its propagation through a vacuum, the wave model is used. Total for Question 1: 10 (a) State three key observations from the photoelectric e ect. [3] Solution:
PDF NSW Education Standards Authority questions and short-answer questions for each of the modules, Advanced Mechanics, ... Quantum Model PH12-5, PH12-6, PH12-14 4-5 Mod 7 - 7* 1 Mod 7 Light: Quantum Model PH12-5, PH12-6, PH12-14 5-6 ... Mod 8 Quantum Mechanical Nature of the Atom PH12-4, PH12-6, PH12-15

0 Response to "39 5.3 physics and the quantum mechanical model worksheet answers"
Post a Comment