40 emission spectra and energy levels worksheet
2 Emission Spectra Energy Levels and Spectral Charts-1.pdf... Thus, emission spectra are experimental proofthat electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in thebrightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found? 3. How do electrons become "excited"? 4. Microsoft Word - Emission Spectra and Energy Levels Worksheet ... When visible light energy from a spectrum tube is passed through a diffraction grating, a bright line spectrum, or line-emission spectrum is produced.
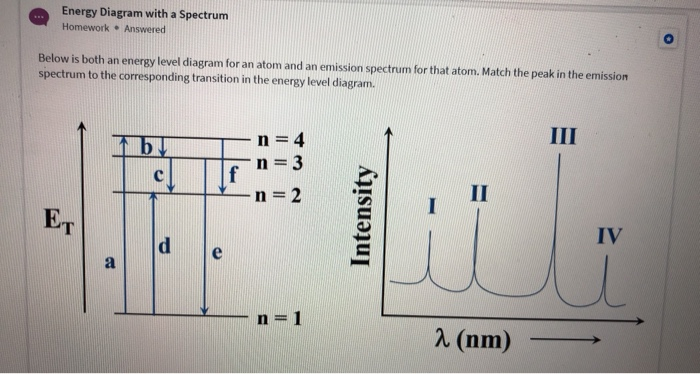
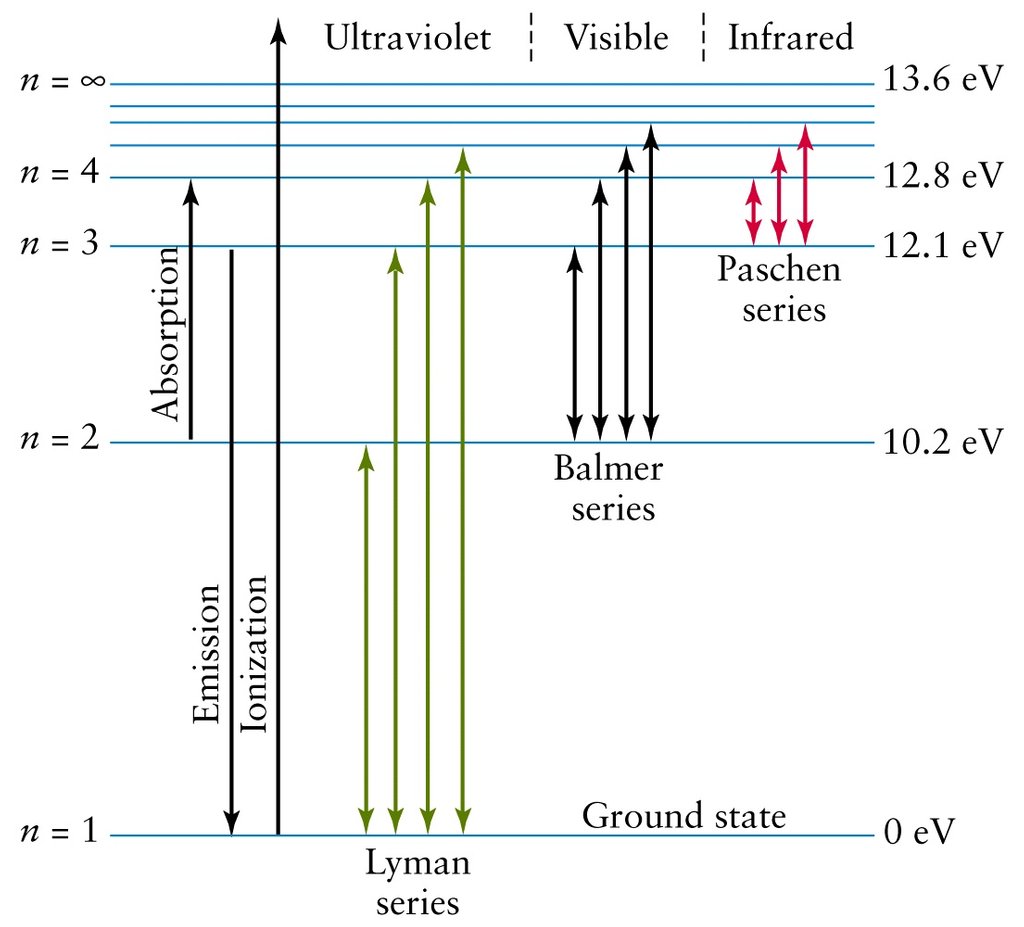
PDF Worksheet 1.3 Emission spectra and electron configurations No. Question Answer 1In which region of the electromagnetic spectrum would you find lines of the greatest energy—ultraviolet, visible or infrared? 2On the energy level diagram show the electron transitions that would result in the series of lines that are seen in the visible region of the hydrogen emission spectrum.

Emission spectra and energy levels worksheet
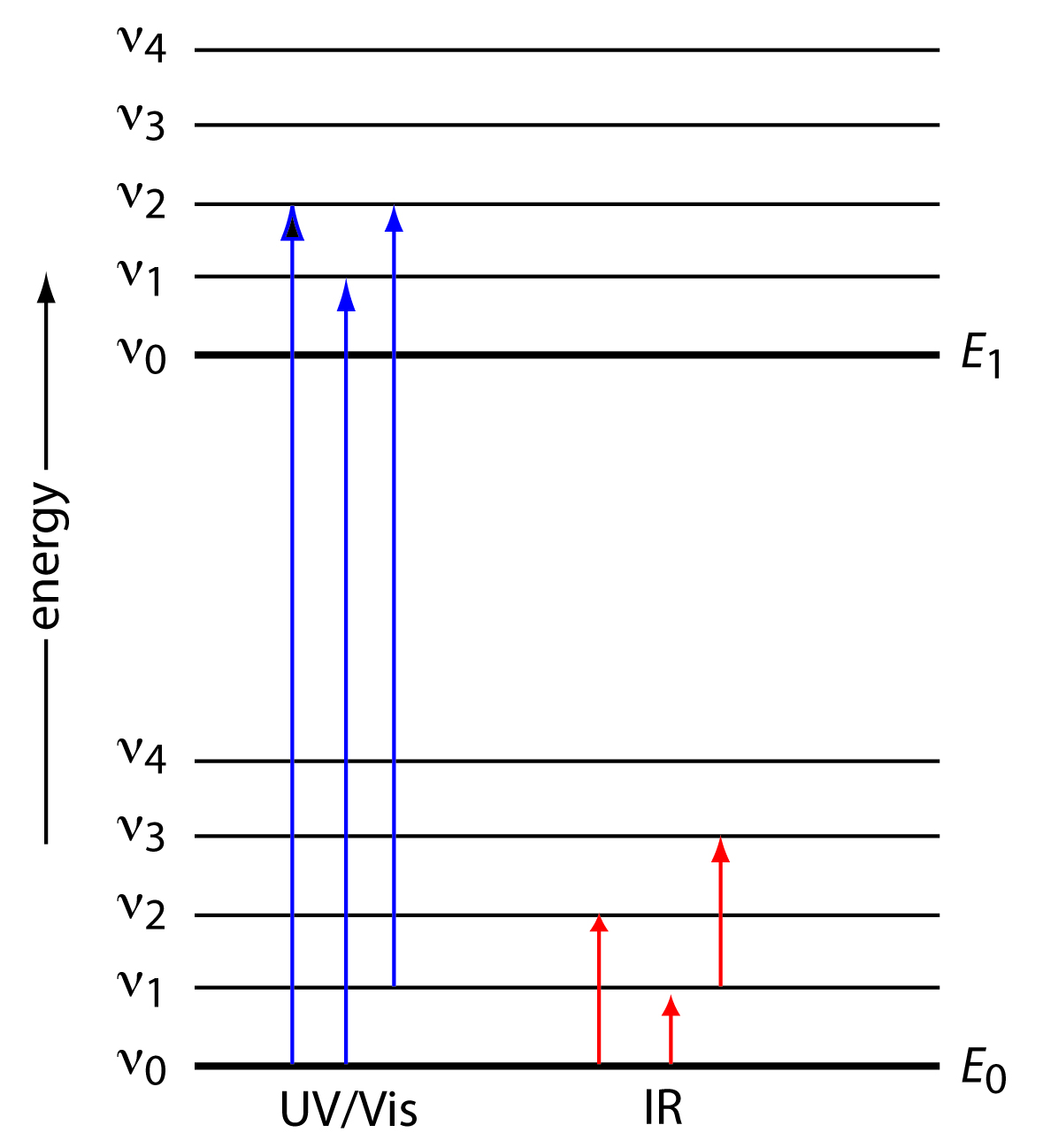
Educator Guide: Using Light to Study Planets | NASA/JPL Edu Have student groups define and differentiate between continuous, absorption and emission spectra using the attached student worksheet and figures before beginning construction of their spectrometer. From the top corner of the cereal box, cut a line the same as the radius of the CD down each face of the box at a 60-degree angle. Chemistry 101 8-ATOMIC EMISSION SPECTRA excited absorb emit visible. relationship between the energy and the frequency and wavelength is given by the following equation: E final- E initial= ∆E = hυ = hc/λ (2) The quantity his known as Planck's constant and is equal to 6.626 x 10-34J ⋅ s (J = joule, s = second). The emission spectrum consists of discrete lines corresponding to the differences in PDF Emission spectra worksheet answers - App Checar Emission spectra and energy levels worksheet answers. Worksheet emission and absorption spectra answers. 5.3 atomic emission spectra worksheet answers. Supposing, we had a sample of a chemical product, but did not know what chemical was. How could we know? One way BEA would be to give a small sample of the chemical product and to burn it.
Emission spectra and energy levels worksheet. PDF Electron Energy and Light Key His model included electrons orbiting the nucleus at specific energy levels. Electrons absorb energy from various sources (electricity) when they move from lower energy levels (ground state) to higher energy levels (excited states). Enerw is released as electrons return to their lower energy levels. 18. Educator Guide: Using Light to Study Planets | NASA/JPL Edu Have student groups define and differentiate between continuous, absorption and emission spectra using the attached student worksheet and figures before beginning construction of their spectrometer. From the top corner of the cereal box, cut a line the same as the radius of the CD down each face of the box at a 60-degree angle. PDF Flame Test Lab Activity Key - University of South Florida wavelength of 459 nm. What is the energy content, in joules, of this photon? (-34 8)( )-19-7-19 6.33 x 10 J s 3.00 x 10 m s = = 4.33 x 10 J 4.59 x 10 m = 4.33 x 10 J E E ⋅ 3. Emission spectrums are produced from the release of light by electrons in varying energy levels. The following is an emission spectrum of hydrogen gas. Calculate the Classwork and Homework Handouts - Penfield Central School District weekly 3 - homework assignment (doc 30 kb) valence electrons worksheet (docx 21 kb) electron configuration worksheet (docx 39 kb) bohr and electron configuration warm up classwork handout (doc 24 kb) bright line spectra warm up worksheet (docx 55 kb) bright line spectra worksheet (docx 227 kb) bright line spectrum of hydrogen and helium worksheet …
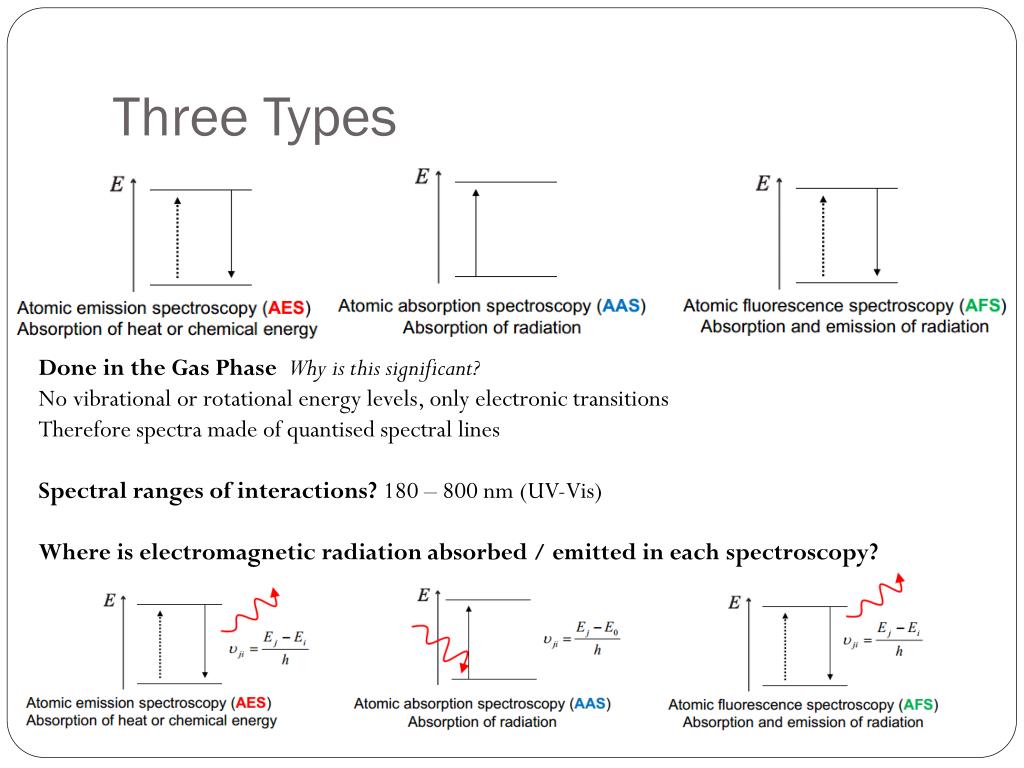
Solved Atomic Emission Spectra Worksheet Part I: | Chegg.com Atomic Emission Spectra Worksheet Part I: Calculations of Electron Energy Levels (E.) in the Bohr Atom of Hydrogen bebe Each shell in the Bohr atom has a positive integer value "n" that represents an energy level for that orbit. Journal of Chemical Education | Vol 99, No 3 Evaluation of Photo-oxidation in Full-Fat Milk under Different Light Spectra: A Laboratory Experiment in Food Chemistry. Aili Wang*, Sihui Ma, Yeyuan Zhu, Liang Zou, and ; Gang Zhao; ... Methane Combustion Energy. Mark E. Wolf, J. … Spectra Of Elements Worksheet Answers - Google Groups The emission spectrum consists of discrete lines corresponding to the differences in energy levels characteristic of and unique along the atoms of the element. Emission Spectra of 10 Elements Answer Key p5 Have students examine the. Photon of radiation that shows up bow this range line-emission spectrum. Worksheet 1 contains the spectra for 7 ... Radioactive Isotope: Definition & Uses - Study.com 31.10.2021 · Radioactive Isotopes: unstable nuclei that radiate radioactive energy until isotope becomes stable Medical Use : radiation therapy, tumor detection, organ imaging Food Safety Use : irradiating to ...
PDF Emission spectra and energy levels worksheet answers questions pdf answer Emission spectra and energy levels worksheet answers questions pdf answer The tube is filled with a gas. If the photon energy does not correspond to the difference between two energy levels then the photon will not be absorbed (it can still be scattered). Graph templates for all types of graphs - Origin Multi-panel plot shows experimental x-ray emission (XES) and absorption (XAS) spectra. The graph contains seven layers. The upper and lower-right layers are grouped XES and XAS line plots, one with an inset plot. The four layers on the lower-left are X-axis-linked color fill contours. All layers can be resized and repositioned flexibly. Student Worksheet: Graphing Spectra - NASA Student Worksheet: Graphing Spectra Solution for Graphing Spectra Student Worksheet, Part II The graphical representation should include all visible lines shown in the color specrum. The continuum should rise gradually from 4000 angstroms, and remain fairly constant through blue, and decrease slightly in green portion of the spectrum. Quiz & Worksheet - Line Emission Spectra | Study.com Worksheet 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission wavelengths are possible....
Atomic Emission Spectra ( Read ) | Chemistry - CK-12 Foundation Describes atomic emission spectra and the source of the emission lines. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic. Click here to view We have moved all content for this concept to for better organization. Please update your bookmarks accordingly.
Bohr's Theory of the Hydrogen Atom | Physics II | | Course Hero Figure 1. Niels Bohr, Danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. His many contributions to the development of atomic physics and quantum mechanics, his personal influence on many students and colleagues, and his personal integrity, especially in the face of Nazi oppression, earned him a prominent place in history.
Balmer series - Wikipedia Balmer's formula. Balmer noticed that a single wavelength had a relation to every line in the hydrogen spectrum that was in the visible light region. That wavelength was 364.506 82 nm.When any integer higher than 2 was squared and then divided by itself squared minus 4, then that number multiplied by 364.506 82 nm (see equation below) gave the wavelength of another line …
Emission Spectra Color Teaching Resources | Teachers Pay Teachers Flame Emission Spectroscopy Homework Sheet (Chemistry Revision) by. Rocket Sheets. $1.50. Zip. This Chemistry Worksheet comes with Answers and covers Flame Emission Spectroscopy in Chemical Analysis. Key words include: Ions, Potassium, Copper, Sodium, Calcium, Lithium, Flame Color, Spectrum, Light, Wavelength, Intensity, Concentration and ...
Digital Library - CRDP An effort made to mitigate the crisis and current circumstances forced by the major spread of the novel corona virus. The Ministry of Education and Higher Education (MEHE), the Center for Educational Research and Development (CERD) as well as public and private school administrations and teachers are all collaborating to provide an effective and impactful solution …
Spectra and energy levels | Teaching Resources - Tes 5. Intro to quanta using line spectra and LEDs. Lots of background info for the teacher, questions (+answers!), experiment details and images to use. Follows the 'Advancing Physics' scheme, but equally useful for all specifications. Empty reply does not make any sense for the end user.
Graph templates for all types of graphs - Origin scientific ... Multi-panel plot shows experimental x-ray emission (XES) and absorption (XAS) spectra. The graph contains seven layers. The upper and lower-right layers are grouped XES and XAS line plots, one with an inset plot. The four layers on the lower-left are X-axis-linked color fill contours. All layers can be resized and repositioned flexibly.
Solution for Student Worksheet: Energy Levels in the Atom Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ...
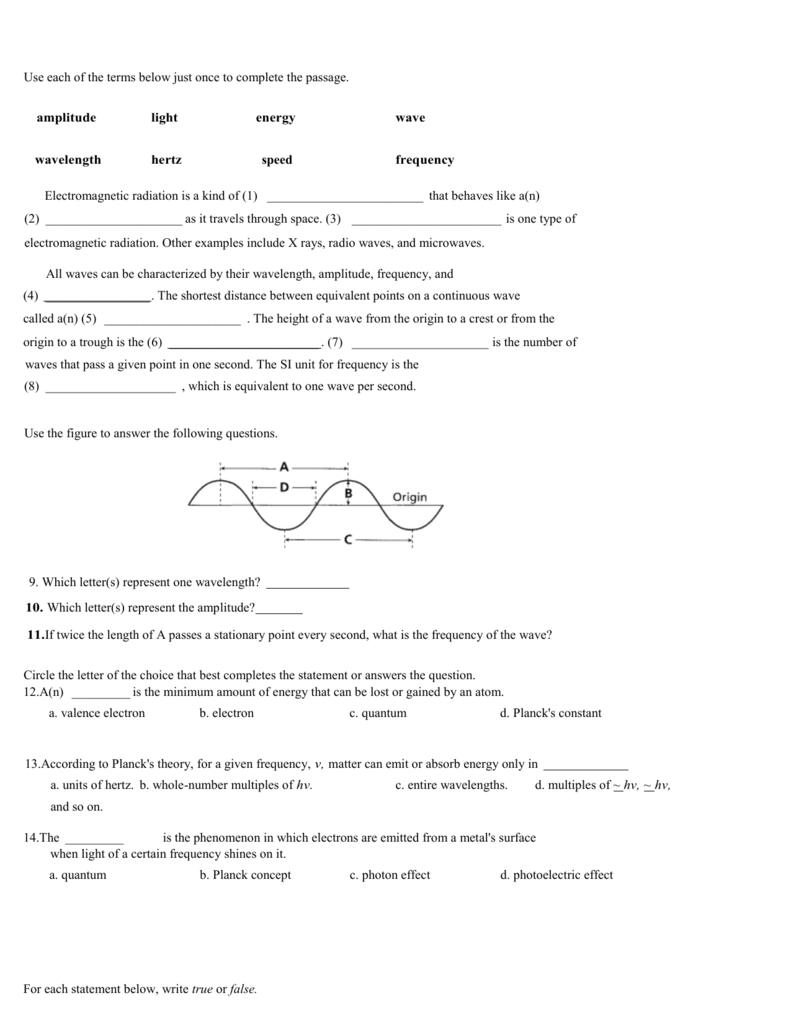
PDF More Practice: Energy, Frequency, Wavelength and the Photoelectric Effect. frequency and work function (Φ) relate to the kinetic energy of the emitted electron. 1 photon ! 1 !! energy of the ejected electron increases as the E difference (between ℎ!!&!Φ) increases. 5. Recalling the information from question 3, what would happen if you were to shine a light of 6.5 eV on the gold surface? How is this the same or ...
PDF Practical Laboratory #2: Emission Spectra - Michigan State University This is called an absorption spectrum because the gas is absorbing light at speci c wavelengths. Fig. 2.2 shows examples of a continuous, an emission and an absorption spectrum. Emission Spectra The discrete bright (dark) lines in the emission (absorption) spectrum can be explained by treating light as a photon that is emitted (absorbed) by an
Chemistry Quizzes | Study.com Electron Configurations in Atomic Energy Levels . View Quiz. Electron Configuration Notation . ... Line Emission Spectra . View Quiz. Atomic Absorptions Spectroscopy . ... Quiz & Worksheet for ...
Analyzing Spectra - physics.weber.edu Consider just four of the energy levels in a certain atom, as shown in this diagram: Assume an emission spectrum and draw arrows indicating the possible transitions. How many spectral lines will result from all possible transitions among these levels? Which transition corresponds to the highest frequency (shortest wavelength) light emitted?
Chemistry Quizzes | Study.com Interested in seeing how well you know a particular chemistry concept? Take Study.com's brief multiple-choice quiz. Obtain rapid feedback and results to …
BIOLOGY H - Essay Help 07.03.2022 · Which way does energy flow and how does eating an organism result in energy transfer? Aldosteronismes body fluid levels by directly promoting the reabsorption of O amino acids O glucose O water O sodium ions; Kindly give ,quick , correct and the most accurate answers. Thank you.i will give helpful rating. 13.
Emission Spectra and Energy Levels Worksheet KEY.doc Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: Look at the spectrum and ...







0 Response to "40 emission spectra and energy levels worksheet"
Post a Comment