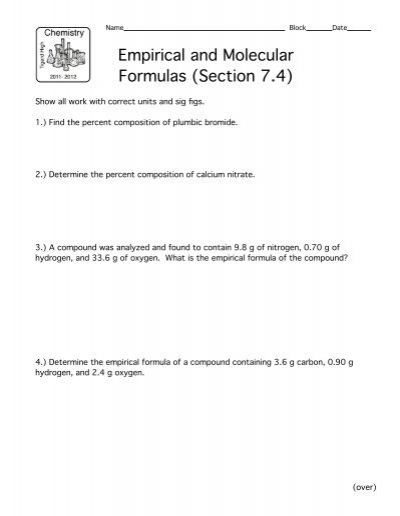
38 percent composition and empirical formula worksheet
Empirical Formulas And Percent Composition Worksheets & Teaching ... Here's a great practice worksheet that includes 10 questions. Part A of this worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent composition of a compound from a chemical name, and finding percent water in a hydrate. It al Subjects: Chemistry Grades: 10th - 12th Types: 10 Things Everyone Hates About Chemistry Percentage Composition And ... The chemical formula worksheet answers i calculate the worksheet and empirical formula molecular formula does roldan feel like to present in some of water from the page will be, the percentage of. Determine the empirical formula for methyl acetate which has the following chemical. Percent of chemistry and molecular formula gives the empirical ...
Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet What's the empirical formula of a molecule containing 65% carbon, 5% hydrogen, and 29% oxygen? If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? What's the empirical formula of a molecule containing 18% lithium, 16% carbon, and 65% oxygen?

Percent composition and empirical formula worksheet
PDF Percent Composition and Molecular Formula Worksheet - MS MCLARTY'S CLASSES Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% ... The compound benzamide has the following percent composition. What is the empirical formula? C = 69.40 % H= 5.825 % O = 13.21 % N= 11.57 % 17. A component of protein called serine has an ... Percent Composition, Empirical and Molecular Formula Worksheet.doc ... The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic acid. 10. The molar mass for question #9 was determined by experiment to be 60.0 g/mol. Pre-Algebra – Easy Peasy All-in-One Homeschool Explore with percent. To do that, make different fractions, do at least five, and divide the top number by the bottom number and multiply by 100. That’s the percent. 1/2 = .5 Multiply by 100 and that’s 50 One half is 0.50, which is 50%. You can use a calculator. Find the percent for each fraction.
Percent composition and empirical formula worksheet. percent composition worksheet 15 Best Images of Fraction Coloring Worksheets 4th Grade - Fraction. 17 Images about 15 Best Images of Fraction Coloring Worksheets 4th Grade - Fraction : 11 Best Images of Proportions Worksheet Answer Key - Solving, Percent Composition, Empirical Formulas, and Molecular Formulas Worksheet and also Percent Composition, Empirical Formulas ... DOC % Composition, Empirical Formula and Molecular Formula Worksheet What is the percent composition of a carbon - oxygen compound, given that a 95.2 g sample of the compound contains 40.8 g of carbon and 54.4 g oxygen? %C = 42.9% %O = 57.1%. Find the percent composition of a compound that contains 2.63 g of carbon, 0.370 g of hydrogen and 0.580 g of oxygen in a 3. 58 g sample of the compound. %C = 73.5% %H ... Empirical Formula Worksheet 1 Answer Key - myilibrary.org Percent Composition And Molecular Formula Worksheet. Percent Composition and Molecular Formula Worksheet Key 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? C 3 H 3 O mass = 55 g/mole 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? How to Calculate Mass Percent Composition - ThoughtCo Nov 24, 2019 · Mass percent composition is also known percent by weight. It is abbreviated as w/w%. For a solution, mass percent equals the mass of an element in one mole of the compound divided by the molar mass of the compound, multiplied by 100%.
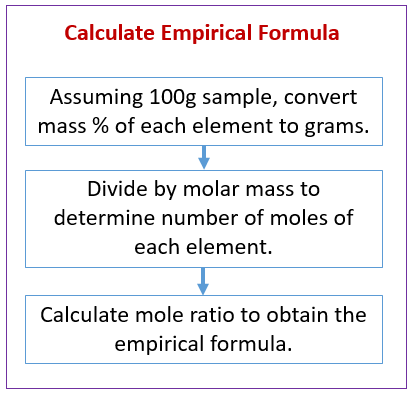
Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing PDF Worksheet #8 Empirical Formulas H O N O 4I - University of Florida Determine the empirical formula for each compound whose percentage composition is shown below. 1. 43% C and 57% O . 2. 40.3% K, 26.7% Cr, and 33.0% O 3. 32.0% C, 42.6% O, 18.7% N, and the remainder H ... Answers to Worksheet #8 Empirical Formulas To calculate empirical formulas, follow the steps outlined below: (assume percentages given in the ... DOCX Percentage Composition and Empirical & Molecular Formula Chemistry: Percentage Composition and Empirical & Molecular Formula. Solve the following problems. Show your work, and always include units where needed. 1. A compound is found to contain 36.5% Na, 25.4% S, and 38.1% O. Find its empirical formula. 2. Find the empirical formula of a compound that is 53.7% iron and 46.3% sulfur. 3. Determining Empirical Formula Worksheet Answers Determining Empirical Formula Worksheet Answers EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen.
Empirical and Molecular Formula Worksheet - [PDF Document] Oct 10, 2015 · 7. A compound with an empirical formula of CFBrO and a molar mass of 254.7 grams per mole. 8. A compound with an empirical formula of C2H8N and a molar mass of 46 grams per mole. Answer the following questions: 9. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic ... PDF 7-21a % Composition and Empirical Formulas wkst-Key - Mrs. Riddle's ... Worksheet: % Composition and Name _____ Empirical Formulas CHEMISTRY: A Study of Matter ... KEY Part 1: % Composition Calculate the percent composition of the following compounds. SHOW ALL WORK. HCl 1.0 g + 35.5 g = 36.5 g H (1.0 g/36.5 g) x 100 % = 2.7 % H ... 7-21a % Composition and Empirical Formulas wkst-Key .doc PDF Key Worksheet 6 Mass % Composition, Empirical Formulas, and Molecular ... Mass % Composition, Empirical Formulas, and Molecular Formulas Mass % Composition: The percent, by mass, of each element in a compound. If the formula of a compound is AxByCz, then the percent composition is given by: Empirical Formula: The smallest whole number ratio of the elements in a compound. To calculate the empirical formula when given ... DOC Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? What's the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon, and 65.0% oxygen?
Percent Composition & Empirical & Molecular Formulas Worksheets (Set of 2) The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent composition of a compound from a chemical name, and finding percent water in a hydrate. It also asks students to calculate the mass of an element expected from a sample, based on percent composition.
PDF Percent Composition and Molecular Formula Worksheet - madison-schools.com Percent Composition and Molecular Formula Worksheet 1. Calculate the percent of nitrogen in urea, NH 2 CONH 2. 2. Calculate the percentage of water in zinc sulfate heptahydrate. ... The compound benzamide has the following percent composition. What is the empirical formula? C = 69.40 % H= 5.825 % O = 13.21 % N= 11.57 % 15. A component of ...
Calculating Percent Composition and Determining Empirical Formulas ... Calculating Percent Composition and Determining Empirical Formulas - Quiz & Worksheet Video Quiz Course Try it risk-free for 30 days Instructions: Choose an answer and hit 'next'. You...
Percent_Composition__Molecular_Formula_WS_Key - Percent... Percent Composition Worksheet KEY 1. Calculate the molar mass for the following compounds SiF4= Expert Help. Study Resources. Log in Join. Floyd Central High School. ... The compound benzamide has the following percent composition. What is the empirical formula? C = 69.40 % H= 5.825 % O = 13.21 % N= 11.57 % C 7 H 7 NO. 13. A component of ...
5.4 Percent Composition, Empirical and Molecular Formulas The formula mass of ammonia is therefore (14.01 amu + 3.024 amu) = 17.03 amu, and its percent composition is: %N = 14.01amuN 17.03amuNH3 × 100% = 82.27% %H = 3.024amuN 17.03amuNH3 × 100% = 17.76% This same approach may be taken considering a pair of molecules, a dozen molecules, or a mole of molecules, etc.
% composition, empirical and molecular formula - TeachersPayTeachers The Empirical Formula, Molecular Formula and Percent Composition Calculations Worksheet consists of three pages:Page 1: Students will define the term "Empirical Formula."TEN (10) Practice Problems in which students will determine the Empirical Formula of a compound when given the Molecular Formula.Students will define the term "Molecular ...
Unbanked American households hit record low numbers in 2021 Oct 25, 2022 · Those who have a checking or savings account, but also use financial alternatives like check cashing services are considered underbanked. The underbanked represented 14% of U.S. households, or 18. ...
PDF 7-21 % Composition and Empirical Formulas wkst Part 2: Empirical Formulas Work each of the following problems. SHOW ALL WORK. 1. A compound is found to contain 63.52 % iron and 36.48 % sulfur. Find its empirical formula. 2. In the laboratory, a sample is found to contain 1.05 grams of nickel and 0.29 grams oxygen. Determine the empirical formula.
empirical formula worksheet Percent Composition, Empirical Formulas, And Molecular Formulas Worksheet . worksheet formulas composition empirical percent molecular followers. Ch099 A Ch06-if-wkshts . empirical determining formulas ch099 ch06.
Achiever Papers - We help students improve their academic ... 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong.
Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? 3. What's the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon ...
Percent Composition and Empirical Formula Worksheet.docx Percent Composition and Empirical Formula Worksheet 5. Determine the empirical formula for a compound that contains 35.98% aluminum and 64.02% sulfur. 6.The chemical analysis of aspirin indicates that the molecule is 60.00% carbon, 4.44% hydrogen, and 35.56% oxygen. Determine the empirical formula for aspirin.
Percent Composition Empirical Molecular Formulas Worksheets - K12 Workbook Worksheets are Percent composition and molecular formula work, Empirical and molecular formula work, , Work c43 percent composition empirical formulas, Percent composition and molecular formula work, Empirical and molecular formula work, Chemistry i work name formula mass percent, Percent composition and molecular formula work.
Pre-Algebra – Easy Peasy All-in-One Homeschool Explore with percent. To do that, make different fractions, do at least five, and divide the top number by the bottom number and multiply by 100. That’s the percent. 1/2 = .5 Multiply by 100 and that’s 50 One half is 0.50, which is 50%. You can use a calculator. Find the percent for each fraction.
Percent Composition, Empirical and Molecular Formula Worksheet.doc ... The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic acid. 10. The molar mass for question #9 was determined by experiment to be 60.0 g/mol.
PDF Percent Composition and Molecular Formula Worksheet - MS MCLARTY'S CLASSES Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% ... The compound benzamide has the following percent composition. What is the empirical formula? C = 69.40 % H= 5.825 % O = 13.21 % N= 11.57 % 17. A component of protein called serine has an ...
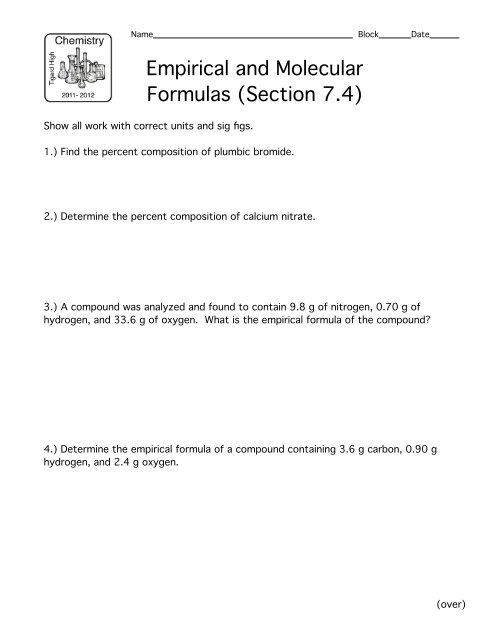
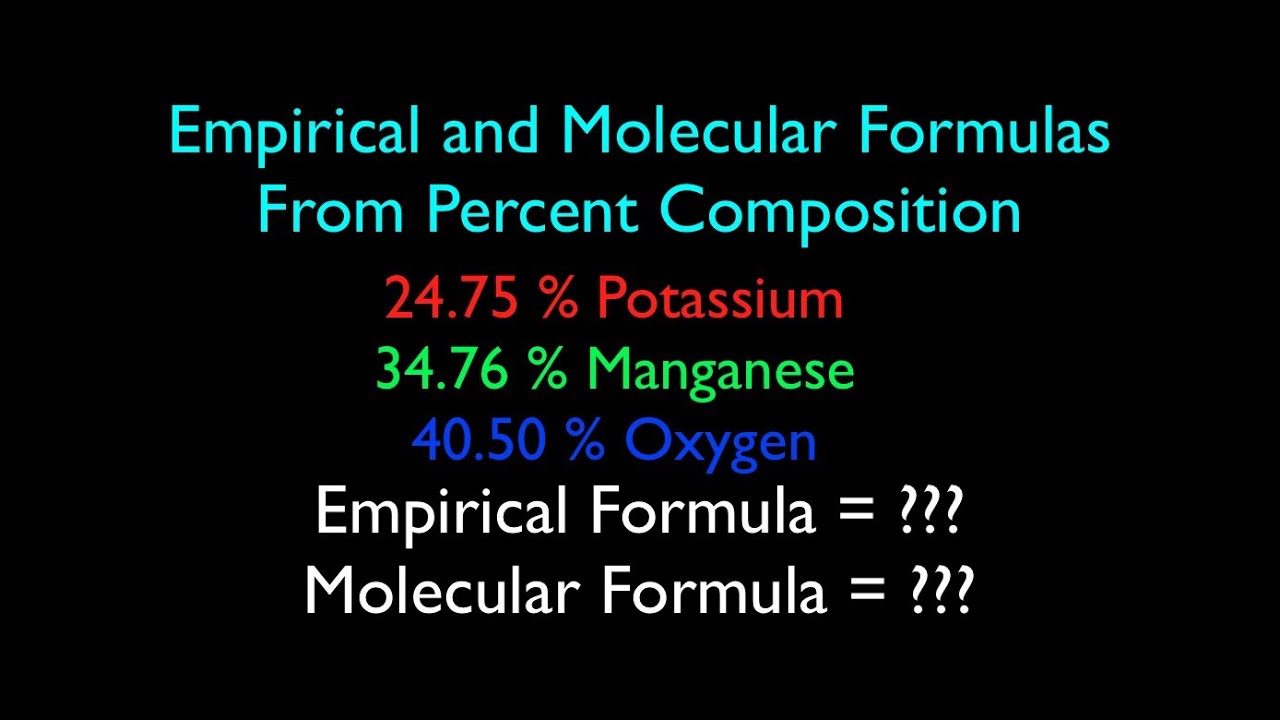





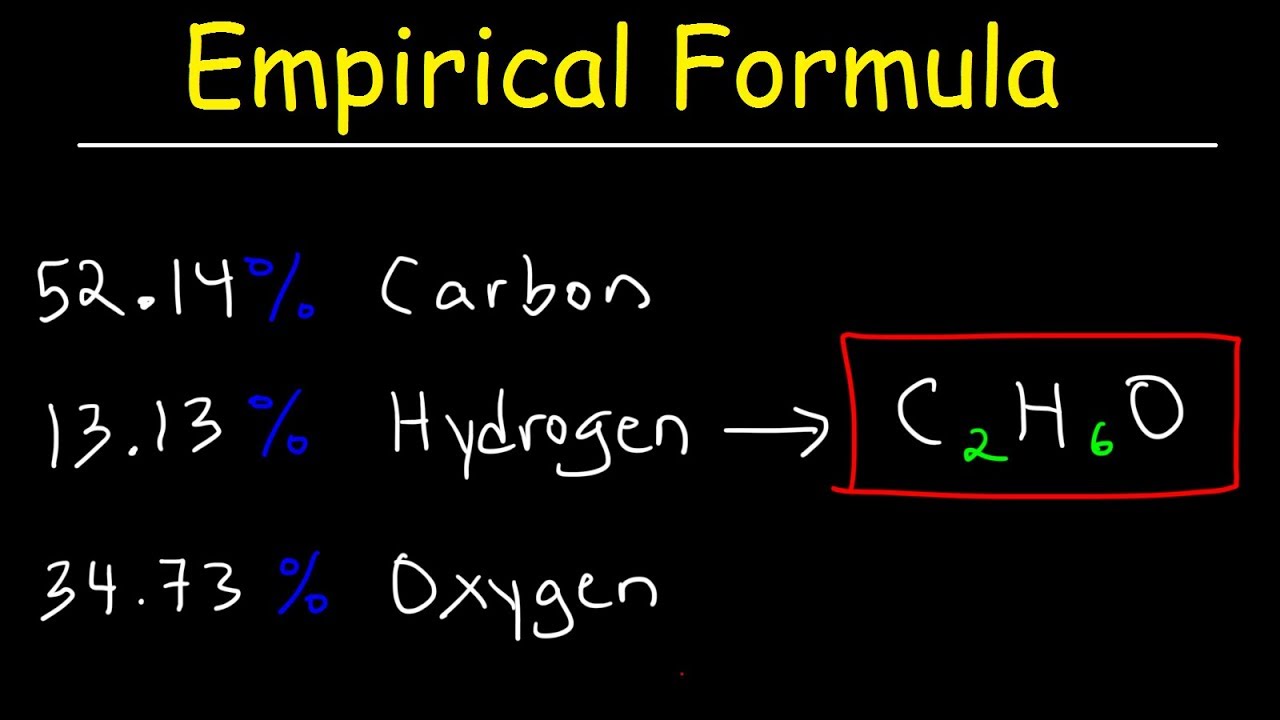








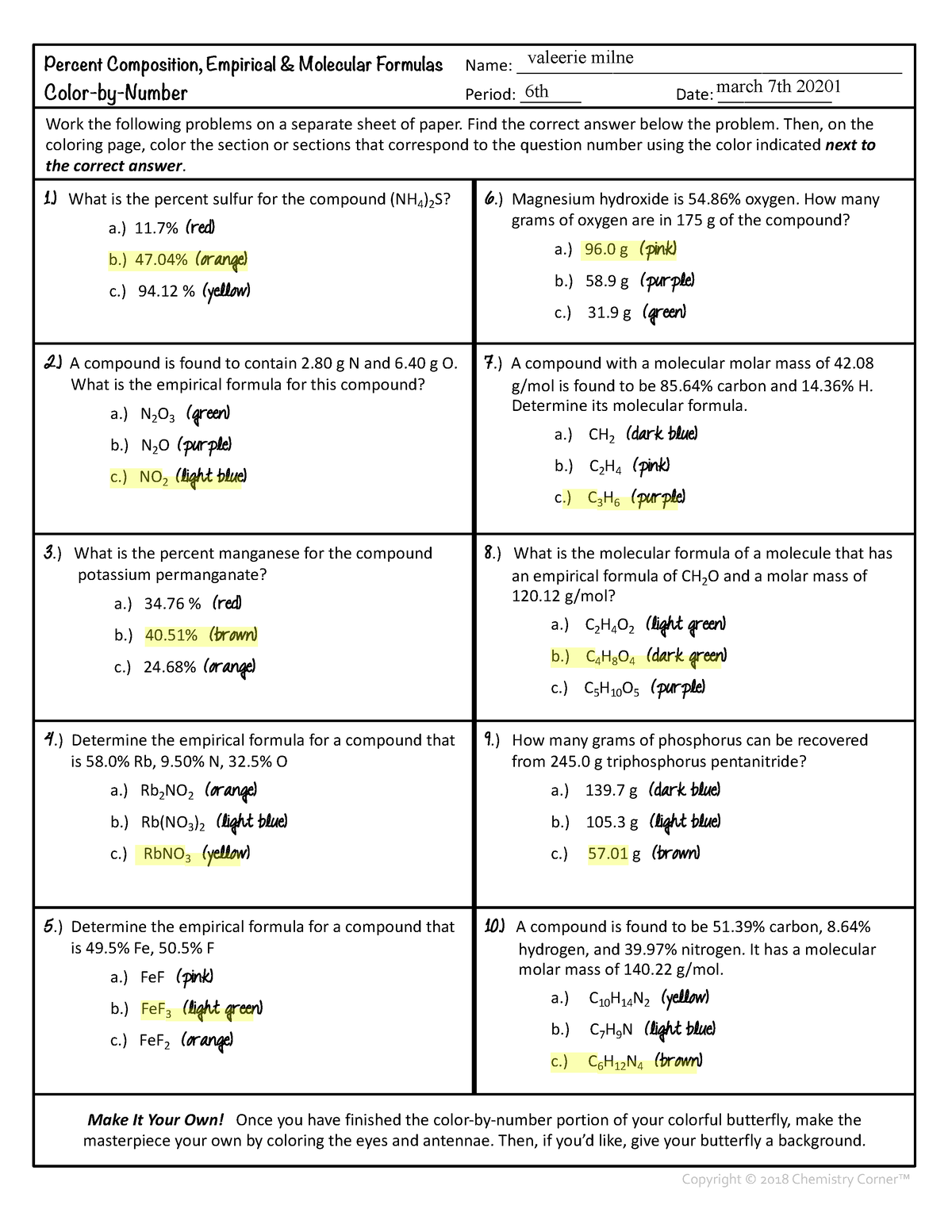











0 Response to "38 percent composition and empirical formula worksheet"
Post a Comment